NAD metabolism modulates inflammation and mitochondria function in diabetic kidney disease
- PMID: 37429506
- PMCID: PMC10413283
- DOI: 10.1016/j.jbc.2023.104975
NAD metabolism modulates inflammation and mitochondria function in diabetic kidney disease
Abstract
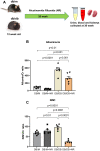
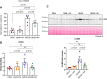
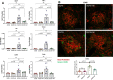
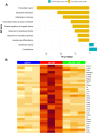
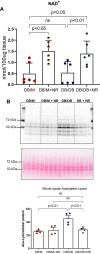
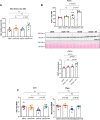
Diabetes mellitus is the leading cause of cardiovascular and renal disease in the United -States. Despite the beneficial interventions available for patients with diabetes, there remains a need for additional therapeutic targets and therapies in diabetic kidney disease (DKD). Inflammation and oxidative stress are increasingly recognized as important causes of renal diseases. Inflammation is closely associated with mitochondrial damage. The molecular connection between inflammation and mitochondrial metabolism remains to be elucidated. Recently, nicotinamide adenine nucleotide (NAD+) metabolism has been found to regulate immune function and inflammation. In the present studies, we tested the hypothesis that enhancing NAD metabolism could prevent inflammation in and progression of DKD. We found that treatment of db/db mice with type 2 diabetes with nicotinamide riboside (NR) prevented several manifestations of kidney dysfunction (i.e., albuminuria, increased urinary kidney injury marker-1 (KIM1) excretion, and pathologic changes). These effects were associated with decreased inflammation, at least in part via inhibiting the activation of the cyclic GMP-AMP synthase-stimulator of interferon genes (cGAS-STING) signaling pathway. An antagonist of the serum stimulator of interferon genes (STING) and whole-body STING deletion in diabetic mice showed similar renoprotection. Further analysis found that NR increased SIRT3 activity and improved mitochondrial function, which led to decreased mitochondrial DNA damage, a trigger for mitochondrial DNA leakage which activates the cGAS-STING pathway. Overall, these data show that NR supplementation boosted NAD metabolism to augment mitochondrial function, reducing inflammation and thereby preventing the progression of diabetic kidney disease.
Keywords: NAD; cGAS-STING; diabetic kidney disease; inflammation, diabetes; mitochondria; mitochondrial DNA damage; sirtuin 3.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures


















References
-
- Thomas M.C., Cooper M.E., Zimmet P. Changing epidemiology of type 2 diabetes mellitus and associated chronic kidney disease. Nat. Rev. Nephrol. 2016;12:73–81. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

