Ube4A maintains metabolic homeostasis and facilitates insulin signaling in vivo
- PMID: 37429524
- PMCID: PMC10368927
- DOI: 10.1016/j.molmet.2023.101767
Ube4A maintains metabolic homeostasis and facilitates insulin signaling in vivo
Abstract
Objective: Defining the regulators of cell metabolism and signaling is essential to design new therapeutic strategies in obesity and NAFLD/NASH. E3 ubiquitin ligases control diverse cellular functions by ubiquitination-mediated regulation of protein targets, and thus their functional aberration is associated with many diseases. The E3 ligase Ube4A has been implicated in human obesity, inflammation, and cancer. However, its in vivo function is unknown, and no animal models are available to study this novel protein.
Methods: A whole-body Ube4A knockout (UKO) mouse model was generated, and various metabolic parameters were compared in chow- and high fat diet (HFD)-fed WT and UKO mice, and in their liver, adipose tissue, and serum. Lipidomics and RNA-Seq studies were performed in the liver samples of HFD-fed WT and UKO mice. Proteomic studies were conducted to identify Ube4A's targets in metabolism. Furthermore, a mechanism by which Ube4A regulates metabolism was identified.
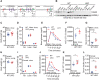
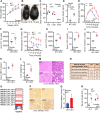
Results: Although the body weight and composition of young, chow-fed WT and UKO mice are similar, the knockouts exhibit mild hyperinsulinemia and insulin resistance. HFD feeding substantially augments obesity, hyperinsulinemia, and insulin resistance in both sexes of UKO mice. HFD-fed white and brown adipose tissue depots of UKO mice have increased insulin resistance and inflammation and reduced energy metabolism. Moreover, Ube4A deletion exacerbates hepatic steatosis, inflammation, and liver injury in HFD-fed mice with increased lipid uptake and lipogenesis in hepatocytes. Acute insulin treatment resulted in impaired activation of the insulin effector protein kinase Akt in liver and adipose tissue of chow-fed UKO mice. We identified the Akt activator protein APPL1 as a Ube4A interactor. The K63-linked ubiquitination (K63-Ub) of Akt and APPL1, known to facilitate insulin-induced Akt activation, is impaired in UKO mice. Furthermore, Ube4A K63-ubiquitinates Akt in vitro.
Conclusion: Ube4A is a novel regulator of obesity, insulin resistance, adipose tissue dysfunction and NAFLD, and preventing its downregulation may ameliorate these diseases.
Keywords: APPL1; Insulin/Akt signaling; NAFLD; Obesity; Ube4A; Ubiquitination.
Copyright © 2023 The Author(s). Published by Elsevier GmbH.. All rights reserved.
Conflict of interest statement
Declaration of competing interest B.T. has been an advisor or consultant for Alimentiv, Allergan, Allysta, Alnylam, Amgen, Arrowhead, Axcella, Boehringer Ingelheim, BMS, Coherus, Cymabay, Enanta, Fortress, Genfit, Gilead, High Tide, HistoIndex, Innovo, Intercept, Ionis, LG Chem, Lipocine, Madrigal, Medimmune, Merck, Mirum, NGM, NovoNordisk, Novus Therapeutics, pHPharma, Sagimet, Target RWE, 89Bio; he has stock options in HepGene; he has institutional research grants from Allergan, BMS, Cirius, Enanta, Genfit, Gilead, Intercept, Madrigal, NGM. B.N.F is a shareholder and a member of the Scientific Advisory Board for Cirius Therapeutics. The other authors do not have any conflict of interest.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

