A lipidated TLR7/8 adjuvant enhances the efficacy of a vaccine against fentanyl in mice
- PMID: 37429853
- PMCID: PMC10333387
- DOI: 10.1038/s41541-023-00694-y
A lipidated TLR7/8 adjuvant enhances the efficacy of a vaccine against fentanyl in mice
Abstract
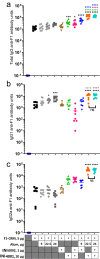
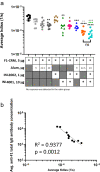
Opioid use disorders (OUD) and opioid-related fatal overdoses are a public health concern in the United States. Approximately 100,000 fatal opioid-related overdoses occurred annually from mid-2020 to the present, the majority of which involved fentanyl or fentanyl analogs. Vaccines have been proposed as a therapeutic and prophylactic strategy to offer selective and long-lasting protection against accidental or deliberate exposure to fentanyl and closely related analogs. To support the development of a clinically viable anti-opioid vaccine suitable for human use, the incorporation of adjuvants will be required to elicit high titers of high-affinity circulating antibodies specific to the target opioid. Here we demonstrate that the addition of a synthetic TLR7/8 agonist, INI-4001, but not a synthetic TLR4 agonist, INI-2002, to a candidate conjugate vaccine consisting of a fentanyl-based hapten, F1, conjugated to the diphtheria cross-reactive material (CRM), significantly increased generation of high-affinity F1-specific antibody concentrations, and reduced drug distribution to the brain after fentanyl administration in mice.
© 2023. The Author(s).
Conflict of interest statement
M.P. is the inventor of patents related to fentanyl haptens, fentanyl hapten conjugates, and methods for making and using the same. J.T.E., D.J.B., and H.G.B. are co-inventors of INI-4001. H.G.B. is a co-inventor of INI-2002. J.T.E., H.G.B., D.J.B., and S.M.M. hold stock options in Inimmune Corporation. The remaining authors declare no competing interests.
Figures




References
-
- Gladden RM, Martinez P, Seth P. Fentanyl law enforcement submissions and increases in synthetic opioid–involved overdose deaths—27 states, 2013–2014. Morb. Mortal. Wkly. Rep. 2016;65:837–843. - PubMed
-
- Ahmad FB, R. L., Sutton P. Provisional drug overdose death counts. National Center for Health Statistics (2022).
-
- Armenian P, Vo KT, Barr-Walker J, Lynch KL. Fentanyl, fentanyl analogs and novel synthetic opioids: a comprehensive review. Neuropharmacology. 2018;134:121–132. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

