Adeno-associated viral vectors for functional intravenous gene transfer throughout the non-human primate brain
- PMID: 37430038
- PMCID: PMC10575780
- DOI: 10.1038/s41565-023-01419-x
Adeno-associated viral vectors for functional intravenous gene transfer throughout the non-human primate brain
Abstract
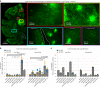
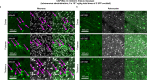
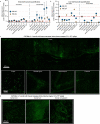
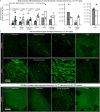
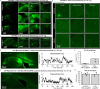
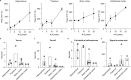
Crossing the blood-brain barrier in primates is a major obstacle for gene delivery to the brain. Adeno-associated viruses (AAVs) promise robust, non-invasive gene delivery from the bloodstream to the brain. However, unlike in rodents, few neurotropic AAVs efficiently cross the blood-brain barrier in non-human primates. Here we report on AAV.CAP-Mac, an engineered variant identified by screening in adult marmosets and newborn macaques, which has improved delivery efficiency in the brains of multiple non-human primate species: marmoset, rhesus macaque and green monkey. CAP-Mac is neuron biased in infant Old World primates, exhibits broad tropism in adult rhesus macaques and is vasculature biased in adult marmosets. We demonstrate applications of a single, intravenous dose of CAP-Mac to deliver functional GCaMP for ex vivo calcium imaging across multiple brain areas, or a cocktail of fluorescent reporters for Brainbow-like labelling throughout the macaque brain, circumventing the need for germline manipulations in Old World primates. As such, CAP-Mac is shown to have potential for non-invasive systemic gene transfer in the brains of non-human primates.
© 2023. The Author(s).
Conflict of interest statement
The California Institute of Technology has filed and licensed patent applications for the work described in this manuscript, with N.C.F., N.G. and V.G. listed as inventors (US patent application no. PCT/US21/46904). V.G. is a co-founder and board member and N.C.F. and N.G. are co-founders and officers of Capsida Biotherapeutics, a fully integrated AAV engineering and gene therapy company. A license for US patent application no. PCT/US21/46904 has been issued to Capsida Biotherapeutics. B.D. is a scientific founder and scientific advisor of Apertura Gene Therapy and is on the scientific advisory board of Tevard Biosciences. B.P.L. is on the scientific advisory board of Patch Biosciences. The remaining authors declare no competing interests.
Figures







Update of
-
Intravenous functional gene transfer throughout the brain of non-human primates using AAV.Res Sq [Preprint]. 2023 Jan 13:rs.3.rs-1370972. doi: 10.21203/rs.3.rs-1370972/v1. Res Sq. 2023. Update in: Nat Nanotechnol. 2023 Oct;18(10):1241-1251. doi: 10.1038/s41565-023-01419-x. PMID: 36789432 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

