Knife's edge: Balancing immunogenicity and reactogenicity in mRNA vaccines
- PMID: 37430088
- PMCID: PMC10394010
- DOI: 10.1038/s12276-023-00999-x
Knife's edge: Balancing immunogenicity and reactogenicity in mRNA vaccines
Abstract
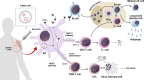
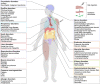
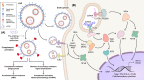
Since the discovery of messenger RNA (mRNA), there have been tremendous efforts to wield them in the development of therapeutics and vaccines. During the COVID-19 pandemic, two mRNA vaccines were developed and approved in record-breaking time, revolutionizing the vaccine development landscape. Although first-generation COVID-19 mRNA vaccines have demonstrated over 90% efficacy, alongside strong immunogenicity in humoral and cell-mediated immune responses, their durability has lagged compared to long-lived vaccines, such as the yellow fever vaccine. Although worldwide vaccination campaigns have saved lives estimated in the tens of millions, side effects, ranging from mild reactogenicity to rare severe diseases, have been reported. This review provides an overview and mechanistic insights into immune responses and adverse effects documented primarily for COVID-19 mRNA vaccines. Furthermore, we discuss the perspectives of this promising vaccine platform and the challenges in balancing immunogenicity and adverse effects.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

