Extended-Release Viloxazine Compared with Atomoxetine for Attention Deficit Hyperactivity Disorder
- PMID: 37430151
- PMCID: PMC10374479
- DOI: 10.1007/s40263-023-01023-6
Extended-Release Viloxazine Compared with Atomoxetine for Attention Deficit Hyperactivity Disorder
Abstract
Background and objective: In our outpatient pediatric and adult psychiatry centers, we reserve psychostimulants for predominantly inattentive attention deficit hyperactivity disorder (ADHD) due to the potential for appetite and growth suppression, insomnia, wear off, exacerbation of mood, anxiety, and tics, or misuse. We utilize extended-release (ER) alpha-2 agonists primarily for hyperactivity/impulsivity but find them less effective for inattention, and they can cause sedation and hypotension. Oftentimes, we need to combine an alpha-2 agonist for behavior with psychostimulants for inattention. We employ atomoxetine or viloxazine ER (VER) for combined ADHD. However, our patients' insurers mandate a trial of generic atomoxetine prior to covering branded VER. The objective of this study was to determine whether pediatric and adult patients taking atomoxetine for DSM-5-TR ADHD combined type would experience improvement in ADHD symptoms following voluntary, open-label switch to VER.
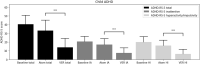
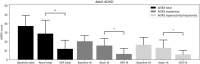
Methods: 50 patients (35 children) received mean doses of atomoxetine 60 mg (25-100 mg once daily) followed by VER 300 mg (100-600 mg once daily) after a 5-day atomoxetine washout. Both atomoxetine and VER were flexibly titrated according to US Food and Drug Administration (FDA) guidelines. The pediatric ADHD-Rating Scale-5 (ADHD-RS-5) and the Adult Investigator Symptom Rating Scale (AISRS) were completed prior to starting atomoxetine, and 4 weeks after treatment with atomoxetine or upon earlier response or discontinuation due to side effects, whichever occurred first; the same protocol was used after treatment with VER. We conducted a blinded, de-identified, retrospective review of charts from these 50 patients in the regular course of outpatient practice. Statistical analysis was performed using a within-subject, 2-tailed t-test with significance level of p < 0.05.
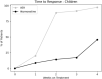
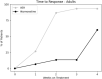
Results: From the baseline total ADHD-RS-5 mean score (40.3 ± 10.3), improvements were greater on VER (13.9 ± 10.2) than atomoxetine (33.1 ± 12.1; t = - 10.12, p < 0.00001) in inattention (t = - 8.57, p < 0.00001) and in hyperactivity/impulsivity (t = - 9.87, p < 0.00001). From the baseline total AISRS mean score (37.3 ± 11.8), improvements were greater on VER (11.9 ± 9.4) than atomoxetine (28.8 ± 14.9; t = - 4.18, p = 0.0009) in inattention (t = - 3.50, p < 0.004) and in hyperactivity/impulsivity (t = - 3.90, p < 0.002). Of patients on VER, 86% reported positive response by 2 weeks versus 14% on atomoxetine. A total of 36% discontinued atomoxetine for side effects, including gastrointestinal (GI) upset (6 patients), irritability (6), fatigue (5), and insomnia (1), versus 4% who discontinued VER due to fatigue. A total of 96% preferred VER over atomoxetine, with 85% (22 out of 26) choosing to taper psychostimulants following stabilization on VER.
Conclusions: Pediatric and adult ADHD patients who have experienced less than optimal response to atomoxetine demonstrate rapid improvement in inattention and in hyperactivity/impulsivity with greater tolerability on extended-release viloxazine.
© 2023. The Author(s).
Conflict of interest statement
Maxwell Z. Price certifies that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript. Richard L. Price has received honoraria from AbbVie, Alkermes, Idorsia, Intra-Cellular Therapies, Janssen, Jazz, Lundbeck, Neuronetics, Otsuka, and Supernus, and discloses no other conflicts of interest in this work.
Figures
Comment in
-
Comment on: "Extended‑Release Viloxazine Compared with Atomoxetine for Attention Deficit Hyperactivity Disorder".CNS Drugs. 2023 Oct;37(10):937-938. doi: 10.1007/s40263-023-01034-3. Epub 2023 Sep 1. CNS Drugs. 2023. PMID: 37656358 No abstract available.
References
-
- Nasser A, Liranso T, Adewole T, et al. A phase III, randomized, placebo-controlled trial to assess the efficacy and safety of once-daily SPN-812 (viloxazine extended-release) in the treatment of attention-deficit/hyperactivity disorder in school-age children. Clin Ther. 2020;42(8):1452–1466. doi: 10.1016/j.clinthera.2020.05.021. - DOI - PubMed
-
- Nasser A, Hull JT, Chaturvedi SA, et al. A phase III, randomized, double-blind, placebo-controlled trial assessing the efficacy and safety of viloxazine extended-release capsules in adults with attention-deficit/hyperactivity disorder. CNS Drugs. 2022;36(8):897–915. doi: 10.1007/s40263-022-00938-w. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

