Extracellular vesicle-associated tyrosine kinase-like orphan receptors ROR1 and ROR2 promote breast cancer progression
- PMID: 37430307
- PMCID: PMC10331971
- DOI: 10.1186/s12964-023-01186-1
Extracellular vesicle-associated tyrosine kinase-like orphan receptors ROR1 and ROR2 promote breast cancer progression
Abstract
Background: Extracellular vesicles (EVs) harbor a plethora of different biomolecules, which they can transport across cells. In cancer, tumor-derived EVs thereby support the creation of a favorable tumor microenvironment. So far, EV uptake and cargo delivery into target cells have been regarded as the main mechanisms for the pro-tumoral function of EVs. To test this hypothesis, we investigated the fate of the oncogenic transmembrane Wnt tyrosine kinase-like orphan receptor 1 and 2 (ROR1, ROR2) delivered via distinct EV subpopulations to breast cancer cells and aimed to unravel their impact on tumor progression.
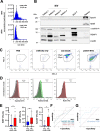
Methods: EVs were isolated by differential ultracentrifugation from cell culture supernatant as well as plasma samples from healthy individuals (n = 27) and breast cancer patients (n = 41). EVs were thoroughly characterized by electron microscopy, nanoparticle tracking analysis, immunoblot, and flow cytometry. ROR transfer to target cells was observed using microscopy-based assays and biodistribution experiments were conducted in syngeneic mice. EV impact on cancer cell migration and invasion was tested in functional assays.
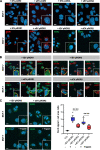
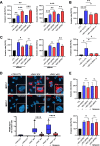
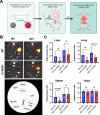
Results: We observed that the supernatant of ROR-overexpressing cells was sufficient for transferring the receptors to ROR-negative cells. Analyzing the secretome of the ROR-overexpressing cells, we detected a high enrichment of ROR1/2 on large and small EVs, but not on large oncosomes. Interestingly, the majority of ROR-positive EVs remained attached to the target cell surface after 24 h of stimulation and was quickly removed by treatment with trypsin. Nonetheless, ROR-positive EVs increased migration and invasion of breast cancer cells, even after chemically inhibiting EV uptake, in dependence of RhoA downstream signaling. In vivo, ROR-depleted EVs tended to distribute less into organs prone for the formation of breast cancer metastases. ROR-positive EVs were also significantly elevated in the plasma of breast cancer patients and allowed to separate them from healthy controls.
Conclusions: The oncogenic Wnt receptors ROR1/2 are transferred via EVs to the surface of ROR-negative cancer cells, in which they induce an aggressive phenotype supporting tumor progression. Video Abstract.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

