Past antihypertensive medication use is associated with lower levels of small vessel disease and lower Aβ plaque stage in the brains of older individuals
- PMID: 37431095
- PMCID: PMC10947144
- DOI: 10.1111/nan.12922
Past antihypertensive medication use is associated with lower levels of small vessel disease and lower Aβ plaque stage in the brains of older individuals
Abstract
Aims: This study assesses the association of antihypertensive medication use on the severities of neuropathological cerebrovascular disease (CVD excluding lobar infarction) in older individuals.
Methods: Clinical and neuropathological data were retrieved for 149 autopsy cases >75 years old with or without CVD or Alzheimer's disease and no other neuropathological diagnoses. Clinical data included hypertension status, hypertension diagnosis, antihypertensive medication use, antihypertensive medication dose (where available) and clinical dementia rating (CDR). Neuropathological CVD severity was evaluated for differences with anti-hypertensive medication usage.
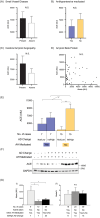
Results: Antihypertensive medication use was associated with less severe white matter small vessel disease (SVD, mainly perivascular dilatation and rarefaction), with a 5.6-14.4 times greater likelihood of less severe SVD if medicated. No significant relationship was detected between infarction (presence, type, number and size), lacunes or cerebral amyloid angiopathy and antihypertensive medication use. Only increased white matter rarefaction/oedema and not perivascular dilation was associated with Alzheimer's pathology, with a 4.3 times greater likelihood of reduced Aβ progression through the brain if white matter rarefaction severity was none or mild. Antihypertensive medication use was associated with reduced Aβ progression but only in those with moderate to severe white matter SVD.
Conclusions: This histopathological study provides further evidence that antihypertensive medication use in older individuals is associated with white matter SVD and not with other CVD pathologies. This is mainly due to a reduction in white matter perivascular dilation and rarefaction/oedema. Even in those with moderate to severe white matter SVD, antihypertensive medication use reduced rarefaction and Aβ propagation through the brain.
Keywords: Alzheimer's disease; antihypertensive medication; cerebrovascular disease; neuropathology; small vessel disease.
© 2023 The Authors. Neuropathology and Applied Neurobiology published by John Wiley & Sons Ltd on behalf of British Neuropathological Society.
Conflict of interest statement
PSS consulted for Biogen Australia and Roche Australia in 2020 and 2021. The other authors have no conflicts of interest. The Editors of Neuropathology and Applied Neurobiology are committed to peer‐review integrity and upholding the highest standards of review. As such, this article was peer‐reviewed by independent, anonymous expert referees, and the authors (including GH) had no role in either the editorial decision or the handling of the paper.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

