Influenza A virus infection during pregnancy causes immunological changes in gut-associated lymphoid tissues of offspring mice
- PMID: 37431584
- PMCID: PMC10435073
- DOI: 10.1152/ajpgi.00062.2023
Influenza A virus infection during pregnancy causes immunological changes in gut-associated lymphoid tissues of offspring mice
Abstract
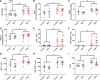
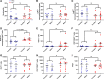
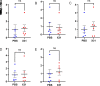
Maternal influenza A virus (IAV) infection during pregnancy can affect offspring immune programming and development. Offspring born from influenza-infected mothers are at increased risk of neurodevelopmental disorders and have impaired respiratory mucosal immunity against pathogens. The gut-associated lymphoid tissue (GALT) represents a large proportion of the immune system in the body and plays an important role in gastrointestinal (GI) homeostasis. This includes immune modulation to antigens derived from food or microbes, gut microbiota composition, and gut-brain axis signaling. Therefore, in this study, we investigated the effect of maternal IAV infection on mucosal immunity of the GI tract in the offspring. There were no major anatomical changes to the gastrointestinal tract of offspring born to influenza-infected dams. In contrast, maternal IAV did affect the mucosal immunity of offspring, showing regional differences in immune cell profiles within distinct GALT. Neutrophils, monocytes/macrophages, CD4+ and CD8+ T cells infiltration was increased in the cecal patch offspring from IAV-infected dams. In the Peyer's patches, only activated CD4+ T cells were increased in IAV offspring. IL-6 gene expression was also elevated in the cecal patch but not in the Peyer's patches of IAV offspring. These findings suggest that maternal IAV infection perturbs homeostatic mucosal immunity in the offspring gastrointestinal tract. This could have profound ramifications on the gut-brain axis and mucosal immunity in the lungs leading to increased susceptibility to respiratory infections and neurological disorders in the offspring later in life.NEW & NOTEWORTHY Influenza A virus (IAV) infection during pregnancy is associated with changes in gut-associated lymphoid tissue (GALT) in the offspring in a region-dependent manner. Neutrophils and monocytes/macrophages were elevated in the cecal patch of offspring from infected dams. This increase in innate immune cell infiltration was not observed in the Peyer's patches. T cells were also elevated in the cecal patch but not in the Peyer's patches.
Keywords: Peyer’s patches; cecal patch; inflammation; influenza A virus; offspring development.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures


References
-
- Jacobsen H, Walendy-Gnirß K, Tekin-Bubenheim N, Kouassi NM, Ben-Batalla I, Berenbrok N, Wolff M, Dos Reis VP, Zickler M, Scholl L, Gries A, Jania H, Kloetgen A, Düsedau A, Pilnitz-Stolze G, Jeridi A, Yildirim AÖ, Fuchs H, Gailus-Durner V, Stoeger C, de Angelis MH, Manuylova T, Klingel K, Culley FJ, Behrends J, Loges S, Schneider B, Krauss-Etschmann S, Openshaw P, Gabriel G. Offspring born to influenza A virus infected pregnant mice have increased susceptibility to viral and bacterial infections in early life. Nat Commun 12: 4957, 2021. doi: 10.1038/s41467-021-25220-3. - DOI - PMC - PubMed
-
- Liong S, Oseghale O, To EE, Brassington K, Erlich JR, Luong R, Liong F, Brooks R, Martin C, O'Toole S, Vinh A, O'Neill LAJ, Bozinovski S, Vlahos R, Papagianis PC, O'Leary JJ, Brooks DA, Selemidis S. Influenza A virus causes maternal and fetal pathology via innate and adaptive vascular inflammation in mice. Proc Natl Acad Sci USA 117: 24964–24973, 2020. doi: 10.1073/pnas.2006905117. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

