Oestrogen therapy for treating pelvic organ prolapse in postmenopausal women
- PMID: 37431855
- PMCID: PMC10335326
- DOI: 10.1002/14651858.CD014592.pub2
Oestrogen therapy for treating pelvic organ prolapse in postmenopausal women
Abstract
Background: Pelvic organ prolapse (POP) is the descent of a woman's uterus, bladder, or rectum into the vagina. It affects 50% of women over 50 years old who have given birth to at least one child, and recognised risk factors are older age, higher number of births, and higher body mass index. This review assesses the effects of oestrogen therapy, alone or in combination with other treatments, on POP in postmenopausal women.
Objectives: To assess the benefits and harms of local and systemic oestrogen therapy in the management of pelvic organ prolapse symptoms in postmenopausal women, and to summarise the principal findings of relevant economic evaluations.
Search methods: We searched the Cochrane Incontinence Specialised Register (up to 20 June 2022), which includes CENTRAL, MEDLINE, two trials registers, and handsearching of journals and conference proceedings. We also checked the reference lists of relevant articles for additional studies.
Selection criteria: We included randomised controlled trials (RCTs), quasi-RCTs, multi-arm RCTs, and cross-over RCTs that evaluated the effects of oestrogen therapy (alone or in combination with other treatments) versus placebo, no treatment, or other interventions in postmenopausal women with any grade of POP.
Data collection and analysis: Two review authors independently extracted data from the included trials using prespecified outcome measures and a piloted extraction form. The same review authors independently assessed the risk of bias of eligible trials using Cochrane's risk of bias tool. Had data allowed, we would have created summary of findings tables for our main outcome measures and assessed the certainty of the evidence using GRADE.
Main results: We identified 14 studies including a total of 1002 women. In general, studies were at high risk of bias in terms of blinding of participants and personnel, and there were also some concerns about selective reporting. Owing to insufficient data for the outcomes of interest, we were unable to perform our planned subgroup analyses (systemic versus topical oestrogen, parous versus nulliparous women, women with versus without a uterus). No studies assessed the effects of oestrogen therapy alone versus no treatment, placebo, pelvic floor muscle training, devices such as vaginal pessaries, or surgery. However, we did identify three studies that assessed oestrogen therapy in conjunction with vaginal pessaries versus vaginal pessaries alone and 11 studies that assessed oestrogen therapy in conjunction with surgery versus surgery alone.
Authors' conclusions: There was insufficient evidence from RCTs to draw any solid conclusions on the benefits or harms of oestrogen therapy for managing POP symptoms in postmenopausal women. Topical oestrogen in conjunction with pessaries was associated with fewer adverse vaginal events compared with pessaries alone, and topical oestrogen in conjunction with surgery was associated with reduced postoperative urinary tract infections compared with surgery alone; however, these findings should be interpreted with caution, as the studies that contributed data varied substantially in their design. There is a need for larger studies on the effectiveness and cost-effectiveness of oestrogen therapy, used alone or in conjunction with pelvic floor muscle training, vaginal pessaries, or surgery, for the management of POP. These studies should measure outcomes in the medium and long term.
Trial registration: ClinicalTrials.gov NCT02906111 NCT03779633 NCT00803335 NCT03032848 NCT02431897 NCT04393194.
Copyright © 2023 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
In accordance with Cochrane's
AT: none known EEJ: is Assistant Managing Editor for Cochrane Incontinence. She did not take any part in the editorial process for this review. SII: none known EBM: none known AK: is an Editor with Cochrane. She did not take any part in the editorial process for this review. RT: none known
Figures

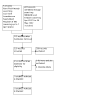










Update of
References
References to studies included in this review
Baracat 2001 {published data only}
-
- Baracat EC, Simões MJ, Soares JM, Haidar MA, Rodrigues de Lima G. Ultrastructural aspects of the postmenopausal endometrium after oral or transdermal estrogen administration. Clinical and Experimental Obstetrics and Gynecology 2001;28(1):26-30. - PubMed
Caruso 2017 {published data only}
-
- Caruso S, Cianci S, Vitale SG, Matarazzo MG, Amore FF, Cianci A. Effects of ultralow topic estriol dose on vaginal health and quality of life in postmenopausal women who underwent surgical treatment for pelvic organ prolapse. Menopause 2017;24(8):900-7. [DOI: 10.1097/GME.0000000000000851] - DOI - PubMed
-
- NCT02906111. Vaginal estriol before and vaginal surgery for prolapse (VSaE) [Effects on vaginal health and quality of life in postmenopausal women using ultra low topic estriol before vaginal surgery for pelvic statics disorders]. clinicaltrials.gov/show/NCT02906111 (first received 19 September 2016).
Chiengthong 2022 {published data only}
-
- Chiengthong K, Ruanphoo P, Chatsuwan T, Bunyavejchevin S. Effect of vaginal estrogen in postmenopausal women using vaginal pessary for pelvic organ prolapse treatment: a randomized controlled trial. International Urogynecology Journal 2022;33(7):1833-8. [DOI: 10.1007/s00192-021-04821-y] - DOI - PubMed
-
- TCTR20180329001. Effect of vaginal estriol 0.03 mg with Lactobacillus acidophilus on incidence of bacterial vaginosis in patient using pessary for treatment of pelvic organ prolapse. thaiclinicaltrials.org/show/TCTR20180329001 (first received 29 March 2018).
Coelho 2020 {published data only}
-
- Coelho SC, Giraldo PC, Brito LG, Teatin Juliato CR. Estrogen use on complications for women treating pelvic organ prolapse with vaginal pessaries (ESTRO-PESS)-a randomized clinical trial (Abstract 007). International Urogynecology Journal 2020;31(Suppl 1):S4. [DOI: 10.1007/s00192-020-04555-3] - DOI - PubMed
-
- RBR-23sqxz. Impact of cream vaginal use on complications in women using vaginal ring in treatment of genital prolapse: a randomized clinical trial [Impacto do uso do creme vaginal nas complicações em mulheres usuárias de anel vaginais no tratamento do prolapso genital: ensaio clínico randomizado] [Impact of estrogen use on complications in women using vaginal pessaries with genital prolapse: a randomized clinical trial [Impacto do uso do estrogênio nas complicações em mulheres usuárias de pessários vaginais com prolapso genital: ensaio clínico randomizado]]. ensaiosclinicos.gov.br/rg/RBR-23sqxz (first received 24 August 2018).
Felding 1992 {published data only}
-
- Felding C, Mikkelsen AL, Clausen HV, Loft A, Larsen LG. Preoperative treatment with oestradiol in women scheduled for vaginal operation for genital prolapse. A randomised, double-blind trial. Maturitas 1992;15(3):241-9. - PubMed
-
- Mikkelsen AL, Felding C, Clausen HV. Clinical effects of preoperative oestradiol treatment before vaginal repair operation. A double-blind, randomized trial. Gynecologic and Obstetric Investigation 1995;40(2):125-8. - PubMed
Karp 2012 {published data only}
-
- Karp D, Jean-Michel M, Peterson T, Johnston Y, Suciu G, Aguilar V, et al. Optimizing post-operative healing following vaginal reconstructive surgery: a triple arm randomized clinical trial of an estradiol-releasing vaginal ring (Abstract 55). Neurourology and Urodynamics 2010;29(6):887-8.
-
- Karp DR, Jean-Michel M, Johnston Y, Suciu G, Aguilar VC, Davila GW. A randomized clinical trial of the impact of local estrogen on postoperative tissue quality after vaginal reconstructive surgery. Female Pelvic Medicine and Reconstructive Surgery 2012;18(4):211-5. [DOI: 10.1097/SPV.0b013e31825e6401] - DOI - PubMed
Marschalek 2021 {published data only}
-
- EUCTR2016-000410-30-AT. Preoperative locally applied oestrogen in postmenopausal women with pelvic organ prolapse: influence on subjective and objective parameters [Preoperative locally applied oestrogen in postmenopausal women with pelvic organ prolapse: changes in subjective and objective outcome: a prospective randomized, double-blind, placebo-controlled study]. www.clinicaltrialsregister.eu/ctr-search/trial/2016-000410-30/results (first received 19 October 2016).
-
- Marschalek ML, Bodner K, Kimberger O, Morgenbesser R, Dietrich W, Obruca C, et al. Sexual function in postmenopausal women with symptomatic pelvic organ prolapse treated either with locally applied estrogen or placebo: results of a double-masked, placebo-controlled, multicenter trial. Journal of Sexual Medicine 2022;19(7):1124-30. [DOI: 10.1016/j.jsxm.2022.04.007] - DOI - PubMed
-
- Marschalek ML, Bodner K, Kimberger O, Morgenbesser R, Dietrich W, Obruca C, et al. Surgical assessment of tissue quality during pelvic organ prolapse repair in postmenopausal women pre-treated either with locally applied estrogen or placebo: results of a double-masked, placebo-controlled, multicenter trial. Journal of Clinical Medicine 2021;10(11):2531. [DOI: 10.3390/jcm10112531] - DOI - PMC - PubMed
-
- Marschalek ML, Bodner K, Kimberger O, Zehetmayer S, Mordenbesser R, Dietrich W, et al. Does preoperative locally applied estrogen treatment facilitate prolapse-associated symptoms in postmenopausal women with symptomatic pelvic organ prolapse? A randomised controlled double-masked, placebo-controlled, multicentre study. BJOG 2021;128(13):2200-8. [DOI: 10.1111/1471-0528.16894] - DOI - PMC - PubMed
-
- NCT03779633. Preoperative oestrogen in postmenopausal women with pelvic organ prolapse [Preoperative locally applied oestrogen in postmenopausal women with pelvic organ prolapse: changes in subjective and objective outcome – a prospective, randomized, double-blind, placebo-controlled, multicenter study]. clinicaltrials.gov/show/NCT03779633 (first received 19 December 2018).
Nunes 2011 {published data only}
-
- Nunes JC, Feldner PC, Castro RA, Nader HB, Sartori MF, Girão MB. Uterine prolapse: evaluation of glycosaminoglycans in postmenopausal women after estrogen therapy. Climacteric 2011;14(1):121-5. - PubMed
Rahn 2014 {published data only}
-
- Good MM, Shi H, Keller P, Roshanravan SM, Word A, Rahn DD. Local estrogen treatment prior to pelvic reconstructive surgery decreases collagenases and elastases in the vaginal wall: a double-blind randomized controlled study in postmenopausal women with prolapse (Abstract 06). Female Pelvic Medicine and Reconstructive Surgery 2013;19(Suppl 5):S47. [DOI: 10.1097/SPV.0b013e3182a5ddf0] - DOI
-
- Good MM, Wai C, Schaffer JI, Corton MM, White A, Shi H, et al. Patient compliance & safety with use of preoperative local estrogen treatment in postmenopausal women with prolapse: Analysis from a double-blind randomized controlled study (Abstract 15). Female Pelvic Medicine and Reconstructive Surgery 2013;19(Suppl 5):S85. [DOI: 10.1097/SPV.0b013e3182a5ddf0] - DOI
-
- NCT01778985. Effect of preoperative estrogen treatment on connective tissues of the pelvic floor (PET) [Effect of preoperative estrogen treatment on connective tissues of the pelvic floor]. clinicaltrials.gov/show/NCT01778985 (first received 29 January 2013).
-
- Rahn DD, Good MM, Shi H, Keller P, Roshanravan SM, Word A. Effects of preoperative local estrogen treatment on connective tissues of the vaginal wall in postmenopausal women with prolapse: a double-blind randomized controlled study (Abstract 05). Female Pelvic Medicine and Reconstructive Surgery 2013;19(Suppl 5):S47. [DOI: 10.1097/SPV.0b013e3182a5ddf0] - DOI
Stipic 2012 {published data only}
Sun 2016 {published data only}
-
- Sun Z, Zhu L, Xu T, Shi X, Lang J. Effects of preoperative vaginal estrogen therapy for the incidence of mesh complication after pelvic organ prolapse surgery in postmenopausal women: is it helpful or a myth? A 1-year randomized controlled trial. Menopause 2016;23(7):740-8. [DOI: 10.1097/GME.0000000000000614] - DOI - PubMed
-
- Zhu L, Sun Z, ChiCTR-TRC-13003481. A Randomized, controlled trial of intravaginal promestriene in women with a risk of mesh exposure associated with transvaginal implantation for pelvic organ prolapse [阴道局部应用雌激素对预防网片重建手术网片暴露的效果研究]. chictr.org.cn/showproj.aspx?proj=6079 (first received 15 August 2013).
Tontivuthikul 2016 {published data only}
-
- Tontivuthikul P, Sanmee U, Wongtra-Ngan S, Pongnarisorn C. Effect of local estrogen cream on vaginal health after pessary use for prolapsed pelvic organ: a randomized controlled trial. Journal of the Medical Association of Thailand 2016;99(7):757-63. - PubMed
Vaccaro 2013 {published data only}
-
- NCT00803335. The effects of local vaginal estrogen in postmenopausal women with pelvic organ prolapse [The effects of local vaginal estrogen in postmenopausal women with pelvic organ prolapse: a randomized control trial]. clinicaltrials.gov/ct2/show/NCT00803335 (first received 05 December 2008).
-
- Vaccaro CM, Crisp CC, Estanol MV, Fellner AN, Mutema GK, Kleeman SD, et al. Administration of vaginal estrogen in women with vaginal atrophy prior to prolapse surgery: an assessment of quality of life outcomes and medication adherence (Abstract 286). International Urogynecology Journal 2011;22(Suppl 3):S1839-40. [DOI: 10.1007/s00192-011-1521-1] - DOI
-
- Vaccaro CM, Crisp CC, Fellner AN, Pauls RN. Impact of preoperative vaginal estrogen on patient quality of life measures: a randomized controlled trial (Abstract 120). Female Pelvic Medicine and Reconstructive Surgery 2012;18(8 Suppl 1):S151-2. [DOI: 10.1097/SPV.0b013e31826abf55] - DOI
-
- Vaccaro CM, Mutema GK, Fellner AN, Crisp CC, Estanol MV, Kleeman SD, et al. Histologic and cytologic effects of vaginal estrogen in women with pelvic organ prolapse: a randomized controlled trial. Female Pelvic Medicine and Reconstructive Surgery 2013;19(1):34-9. [DOI: 10.1097/SPV.0b013e318278cc40] - DOI - PubMed
Verghese 2020 {published data only}ISRCTN46661996
-
- EudraCT2014-000179-18. Local oestrogen treatment in postmenopausal women undergoing pelvic organ prolapse surgery (LOTUS) [Local oestrogen treatment in postmenopausal women undergoing pelvic organ prolapse surgery (LOTUS) – feasibility study]. www.clinicaltrialsregister.eu/ctr-search/trial/2014-000179-18/results (first received 10 March 2015). - PMC - PubMed
-
- ISRCTN46661996. Local oestrogen treatment in postmenopausal women undergoing pelvic organ prolapse surgery [Local oestrogen treatment in postmenopausal women undergoing pelvic organ prolapse surgery (LOTUS) feasibility study]. isrctn.com/ISRCTN46661996 (first received 28 May 2015).
-
- Middleton 2021. Middleton L [Re: study query: randomised controlled trial to investigate the effectiveness of local oestrogen treatment in postmenopausal women undergoing pelvic organ prolapse surgery (LOTUS): a pilot study to assess feasibility of a large multicentre trial [personal communication]]. Email to: E Johnson 2 November 2021. - PMC - PubMed
-
- Verghese TS, Middleton L, Cheed V, Leighton L, Daniels J, Latthe PM. Randomised controlled trial to investigate the effectiveness of local oestrogen treatment in postmenopausal women undergoing pelvic organ prolapse surgery (LOTUS): a pilot study to assess feasibility of a large multicentre trial. BMJ Open 2020;10(9):e025141. [DOI: 10.1136/bmjopen-2018-025141] - DOI - PMC - PubMed
-
- Verghese TS, Middleton LJ, Cheed V, Leighton L, Daniels JP, Latthe PM. A randomised controlled trial to investigate the effectiveness of local oestrogen treatment in postmenopausal women undergoing pelvic organ prolapse surgery (LOTUS)-a pilot study to assess feasibility of a large multi-centre trial (Abstract 026). International Urogynecology Journal 2018;29(Suppl 1):S15-6. [DOI: 10.1007/s00192-018-3752-x] - DOI - PMC - PubMed
References to studies excluded from this review
Barber 2002 {published data only}
-
- Barber MD, Visco AG, Wyman JF, Fantl JA, Bump RC, on behalf of the Continence Program for Women Research Group. Sexual function in women with urinary incontinence and pelvic organ prolapse. Obstetrics and Gynecology 2002;99(2):281-9. - PubMed
Chinthakanan 2019 {published data only}
-
- Chinthakanan O, Wattanayingcharoenchai R, Aimjirakul K, Sarit-Apirak S, Manonai J. Comparison of vaginal moisturizers with ultralow dose estriol & lactobacilluls acidophilus vaginaltablets in pelvic organ prolapse women who were treated with vaginal pessary: a randomized controlled trial (Abstract 144). International Urogynecology Journal 2019;30(Suppl 1):S168. [DOI: 10.1007/s00192-019-04125-2] - DOI
-
- Chinthakanan O, Wattanayingcharoenchai R, Aimjirakul K, Sarit-apirak S, Manonai J. Comparison of vaginal moisturizers with ultra-low dose estriol & lactobacilluls acidophilus vaginal tablets in pelvic organ prolapse women whowere treated with vaginal pessary: a randomized controlled trial (Scientific Salon 144). Female Pelvic Medicine and Reconstructive Surgery 2019;25(5 Suppl 1):S142. [DOI: 10.1097/SPV.0000000000000767] - DOI
-
- Chinthakanan O, TCTR20160621001. Comparison of vaginal moisturizers with ultra-low dose estriol & Lactobacillus acidophilus vaginal tablets in pelvic organ prolapse women who were treated with vaginal pessary: a randomized controlled trial. thaiclinicaltrials.org/show/TCTR20160621001 (first received 20 June 2016).
Chou 2013 {published data only}
-
- NCT01843166. Vaginal estrogen with pessary treatment [Use of vaginal estrogen with pessary treatment of pelvic organ prolapse and urinary incontinence]. clinicaltrials.gov/show/NCT01843166 (first received 30 April 2013).
Jiang 2017 {published data only}
-
- Jiang G, Zhu T, Zhou Y, Ma Q. Effect of puerarin on vaginal connective tissue in patients with pelvic organ prolapse. International Journal of Clinical and Experimental Medicine 2017;10(1):732-8.
Lind 2014 {published data only}
-
- NCT02316249. Vaginal creams to reduce vaginal erosion in pessary users [Vaginal creams for reduction of vaginal discharge and erosion in patients using ring with support or gellhorn pessaries to reduce pelvic organ prolapse]. clinicaltrials.gov/show/NCT02316249 (first received 12 December 2014).
Moalli 2012 {published data only}
-
- NCT01648751. Vaginal estrogen and pelvic floor physical therapy in women with symptomatic mild prolapse [Impact of vaginal estrogen in the treatment of symptomatic mild pelvic organ prolapse with pelvic floor physical therapy]. clinicaltrials.gov/show/NCT01648751 (first received 24 July 2012).
Paraiso 2020 {published data only}
-
- NCT02691936. Comparison of vaginal laser therapy to vaginal estrogen therapy (VeLVET) [A randomized clinical trial comparing vaginal laser therapy to vaginal estrogen therapy in women with genitourinary syndrome of menopause]. clinicaltrials.gov/ct2/show/NCT02691936 (first received 25 February 2016).
-
- Paraiso MF, Ferrando CA, Karram MM, Sokol ER, Rardin CR, Matthews CA, et al. Comparison of vaginal laser therapy to vaginal estrogen therapy (VELVET) for women with genitourinary syndrome of menopause (GSM) (Abstract 62). Female Pelvic Medicine and Reconstructive Surgery 2018;24(5 Suppl 1):S45-6. [DOI: 10.1097/SPV.0000000000000617] - DOI
-
- Paraiso MF, Ferrando CA, Sokol ER, Rardin CR, Matthews CA, Karram MM, et al. A randomized clinical trial comparing vaginal laser therapy to vaginal estrogen therapy in women with genitourinary syndrome of menopause: the VeLVET Trial. Menopause 2020;27(1):50-6. [DOI: 10.1097/GME.0000000000001416] - DOI - PubMed
Valente 2000 {published data only}
-
- Valente M, Dettori C, Feraudo E, Re ME. Pelvic-floor: new therapeutic approach (Abstract IDP66). International Urogynecological Journal 2000;11(Suppl 1):S97.
References to studies awaiting assessment
Pinhat 2013 {published and unpublished data}
-
- NCT03032848. Vaginal estrogens comparative trial on pelvic organ prolapse patients [Randomized, blinded trial comparing vaginal estrogens on pelvic organ prolapse patients]. clinicaltrials.gov/show/NCT03032848 (first received 26 January 2017).
-
- Pinhat E. RE: Study query: Vaginal Estrogens Comparative Trial on Pelvic Organ Prolapse Patients. Email to: E Johnson 18 January 2022.
-
- Pinhat EC. Estudo Comparativo De Cremes Estrogênicos Em Pacientes Com Prolapso Genital [Masters thesis] [Comparative Study of Estrogen Cream in Patients with Genital Prolapse. For information only, English title translated via Google Translate™]. Curitiba (Brazil): Universidade Federal do Paraná, 2017.
Vardy 2003 {published data only}
-
- Vardy MD, Lindsay R, Scotti RJ, Mikhail M, Richart RM, Nieves J, et al. Short-term urogenital effects of raloxifene, tamoxifen, and estrogen. American Journal of Obstetrics and Gynecology 2003;189(1):81-8. - PubMed
-
- Vardy MD, Scotti RJ, Lindsay R, Richart RM, Cruz J, Nieves J, et al. Short term urogenital effects of raloxifene, tamoxifen, and estrogen (Abstract 15). International Urogynecology Journal 2001;12(Suppl 1):S33. - PubMed
Zhu 2014 {published data only}
-
- Zhu L, Sun Z, Lang J. Could vaginal estrogen application prior to surgery reduce mesh exposure in transvaginal pelvic floor reconstruction with mesh? A 2-year randomized controlled trial (Abstract 156). Journal of Minimally Invasive Gynecology 2014;21(Suppl 6):S47. [DOI: ]
-
- Zhu L, Sun Z. Could vaginal estrogen application prior to surgery reduce mesh exposure in transvaginal pelvic floor reconstruction with mesh? A 2-year randomized controlled trial (Abstract PP 46). International Urogynecology Journal 2014;25(Suppl 1):S52. [DOI: 10.1007/s00192-014-2429-3] - DOI
References to ongoing studies
Rahn 2021 {published data only}
-
- NCT02431897. Investigation to minimize prolapse recurrence of the vagina using estrogen (IMPROVE) [Investigation to minimize prolapse recurrence of the vagina using estrogen]. clinicaltrials.gov/show/NCT02431897 (first received 01 May 2015).
-
- Rahn DD, Richter HE, Sung VW, Larsen WI, Hynan LS. Design of a randomized clinical trial of perioperative vaginal estrogen versus placebo with transvaginal native tissue apical prolapse repair (investigation to minimize prolapse repair of the vagina using estrogen: IMPROVE). Female Pelvic Medicine and Reconstructive Surgery 2021;27(1):e227-33. [DOI: 10.1097/SPV.0000000000000899] - DOI - PMC - PubMed
Roovers 2018 {published data only}
-
- EUCTR2017-003144-21-NL. Research into effectiveness and costs concerning the use of (vaginally administered) oestrogen before and after vaginal prolapse surgery, in women after menopause [Onderzoek naar de effectiviteit en kosten van vaginale oestrogeencrème bij vrouwen na de menopauze die een verzakkingsoperatie ondergaan] [Cost-effectiveness of perioperative vaginally administered oestrogen in postmenopausal women undergoing prolapse surgery [Kosteneffectiviteit van perioperatief gebruik van topicale oestrogenen bij postmenopauzale vrouwen die vaginale prolapsoperatie ondergaan]]. www.clinicaltrialsregister.eu/ctr-search/trial/2017-003144-21/NL (first received 28 February 2018).
-
- NL6853, NTR7031. Research into effectiveness and costs concerning the use of oestrogen before and after vaginal prolapse surgery in women after menopause [Cost-effectiveness of perioperative vaginally administered oestrogen in postmenopausal women undergonig prolapse surgery]. trialregister.nl/trial/6853 [Dutch Trial Register (NTR) is undergoing redevelopment, information available via trialsearch.who.int/Trial2.aspx?TrialID=NTR7031] (first received 19 February 2018).
Vincent 2013 {published data only}
-
- NCT01886794. Hormonal status on blood flow and tissue in pelvic organ prolapse [Role of hormonal status on vascularization and vaginal tissue in women with pelvic organ prolapse]. clinicaltrials.gov/show/NCT01886794 (first received 26 June 2013).
Zhu 2020 {published data only}
-
- ChiCTR2000032753. The effect of intravaginal conjugated estrogen on ring pessary use for pelvic organ prolapse: a multicenter randomized, double- blind, placebo controlled, clinical trial [国产环型带膜子宫托联合使用阴道结合雌激素乳膏治疗女性症状性盆腔脏器脱垂的多中心的随机安慰剂对照双盲临床研究]. chictr.org.cn/hvshowproject.aspx?id=32555 (first received 24 March 2020).
-
- NCT04393194. The effect of intra-vaginal conjugated estrogen cream on ring pessary use for pelvic organ prolapse [The effect of intravaginal conjugated estrogen on ring pessary use for pelvic organ prolapse: a multicenter randomized, double- blind, placebo controlled, clinical trial]. clinicaltrials.gov/show/NCT04393194 (first received 19 May 2020).
Additional references
Aluko 2022
-
- Aluko P, Graybill E, Craig D, Henderson C, Drummond M, Wilson EC, et al, on behalf of the Campbell and Cochrane Economics Methods Group. Chapter 20: Economic evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook.
Avery 2004
-
- Avery K, Donovan J, Peters TJ, Shaw C, Gotoh M, Abrams P. ICIQ: a brief and robust measure for evaluating the symptoms and impact of urinary incontinence. Neurourology and Urodynamics 2004;23(4):322-30. - PubMed
Baessler 2009
-
- Baessler K, O'Neill SM, Maher CF, Battistutta D. Australian pelvic floor questionnaire: a validated interviewer-administered pelvic floor questionnaire for routine clinic and research. International Urogynecology Journal 2009;20(2):149-58. - PubMed
Baessler 2019
-
- Baessler K, Mowat A, Maher CF. The minimal important difference of the Australian pelvic floor questionnaire. International Urogynaecology Journal 2019;30(1):115-22. - PubMed
Barber 2001
-
- Barber MD, Kuchibhatla MN, Pieper CF, Bump RC. Psychometric evaluation of 2 comprehensive condition-specific quality of life instruments for women with pelvic floor disorders. American Journal of Obstetrics and Gynecology 2001;185(6):1388-95. - PubMed
Barber 2009
-
- Barber MD, Spino C, Janz NK, Brubaker L, Nygaard I, Nager CW, et al, Pelvic Floor Disorders Network. The minimum important differences for the urinary scales of the pelvic floor distress inventory and pelvic floor impact questionnaire. American Journal of Obstetrics and Gynecology 2009;200(5):580.e1-7. - PMC - PubMed
Bodner‐Adler 2020
-
- Bodner-Adler B, Alarab M, Ruiz-Zapata AM, Latthe P. Effectiveness of hormones in postmenopausal pelvic floor dysfunction – International Urogynecological Association research and development – committee opinion. International Urogynecology Journal 2020;31(8):1577-82. [DOI: 10.1007/s00192-019-04070-0] - DOI - PMC - PubMed
Brookes 2004
-
- Brookes ST, Donovan JL, Wright M, Jackson S, Abrams P. A scored form of the Bristol female lower urinary tract symptoms questionnaire: data from a randomized controlled trial of surgery for women with stress incontinence. American Journal of Obstetrics and Gynecology 2004;191(1):73-82. - PubMed
Bugge 2020
Bump 1996
-
- Bump RC, Mattiasson A, Bø K, Brubaker LP, DeLancey JO, Klarskov P, et al. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. American Journal of Obstetrics and Gynecology 1996;175(1):10-7. - PubMed
Campbell 2020
Cody 2012
Constantine 2017
-
- Constantine ML, Pauls RN, Rogers RR, Rockwood TH. Validation of a single summary score for the prolapse/incontinence sexual questionnaire – IUGA revised (PISQ-IR). International Urogynecology Journal 2017;28(12):1901-7. - PubMed
CRD 2015
-
- Centre for Reviews and Dissemination (CRD). CRD databases: search strategies. 2015. Available at crd.york.ac.uk/crdweb/ (accessed 03 June 2020).
Deeks 2022
-
- Deeks JJ, Higgins JP, Altman DG, editor(s). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
DeLancey 1993
-
- DeLancey JO. Anatomy and biomechanics of genital prolapse. Clinical Obstetrics and Gynecology 1993;36(4):897-909. - PubMed
DeLancey 2002
-
- Delancey JO. Fascial and muscular abnormalities in women with urethral hypermobility and anterior vaginal wall prolapse. American Journal of Obstetrics and Gynecology 2002;187(1):93-8. - PubMed
Dietz 2008
-
- Dietz HP. The aetiology of prolapse. International Urogynecology Journal 2008;19(10):1323-9. - PubMed
Digesu 2005
-
- Digesu GA, Khullar V, Cardozo L, Robinson D, Salvatore S. P-QOL: a validated questionnaire to assess the symptoms and quality of life of women with urogenital prolapse. International Urogynecology Journal 2005;16(3):176-81. - PubMed
Egger 1997
EndNote 2018 [Computer program]
-
- EndNote. Version X8.2. Philadelphia (PA): Clarivate Analytics, 2018.
Evans 1985
-
- Evans DR, Burns JE, Robinson WE, Garrett OJ. The quality of life questionnaire: a multidimensional measure. American Journal of Community Psychology 1985;13(3):305-22.
Frisch 2009
-
- Frisch MB. Quality of Life Inventory Handbook: A Guide for Laypersons, Clients, and Coaches. Minneapolis, MN: NCS Pearson and Pearson, 2009.
GRADEpro GDT [Computer program]
-
- GRADEpro GDT. Version accessed 9 March 2021. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Hagen 2009
-
- Hagen S, Glazener C, Sinclair L, Stark D, Bugge C. Psychometric properties of the pelvic organ prolapse symptom score. BJOG 2009;116(1):25-31. - PubMed
Hagen 2010
-
- Hagen S, Glazener C, Cook J, Herbison P, Toozs-Hobson P. Further properties of the pelvic organ prolapse symptom score: minimally important change and test-retest reliability (Abstract 175). Neurourology and Urodynamics 2010;29(6):1055-6.
Hagen 2011
Hawthorne 1999
-
- Hawthorne G, Richardson J, Osborne R. The assessment of quality of life (AQoL) instrument: a psychometric measure of health-related quality of life. Quality of Life Research 1999;8(3):209-24. - PubMed
Hawthorne 2005
-
- Hawthorne G, Osborne R. Population norms and meaningful differences for the Assessment of Quality of Life (AQoL) measure. Australian and New Zealand Journal of Public Health 2005;29(2):136-42. - PubMed
Haylen 2016
-
- Haylen BT, Maher CF, Barber MD, Camargo SF, Dandolu V, Digesu A, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for pelvic organ prolapse (POP). International Urogynecology Journal 2016;27(2):165-94. - PubMed
Higgins 2011
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2022a
-
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook/archive/v6.2.
Higgins 2022b
-
- Higgins JP, Eldridge S, Li T, editor(s). Chapter 23: Including variants on randomized trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook.
Hyland 1996
-
- Hyland ME, Sodergren SC. Development of a new type of global quality of life scale, and comparison of performance and preference for 12 global scales. Quality of Life Research 1996;5(5):469-80. - PubMed
Ismail 2010
Jelovsek 2006
-
- Jelovsek JE, Barber MD. Women seeking treatment for advanced pelvic organ prolapse have decreased body image and quality of life. American Journal of Obstetrics and Gynecology 2006;194(5):1455-61. - PubMed
Kelleher 1997
-
- Kelleher CJ, Cardozo LD, Khullar V, Salvatore S. A new questionnaire to assess the quality of life of urinary incontinent women. BJOG 1997;104(12):1374-9. - PubMed
Kelleher 2004
-
- Kelleher CJ, Pleil AM, Reese PR, Burgess SM, Brodish PH. How much is enough and who says so? The case of the King's Health Questionnaire and overactive bladder. BJOG 2004;111(6):605-12. - PubMed
Lethaby 2016
Lim 2009
-
- Lim R, Liong ML, Lim KK, Leong WS, Yuen KH. The minimum clinically important difference of the international consultation on incontinence questionnaires (ICIQ-UI SF and ICIQ-LUTSqol). Urology 2019;133:91-5. - PubMed
Lindahl 2014
Løwenstein 2018
-
- Løwenstein E, Møller LA, Laigaard J, Gimbel H. Reoperation for pelvic organ prolapse: a Danish cohort study with 15–20 years' follow-up. International Urogynecology Journal 2018;29(1):119-24. - PubMed
Maher 2017
-
- Maher C, Baessler K, Barber M, Cheon C, Consten E, Cooper K, et al. Pelvic organ prolapse surgery. In: Abrams P, Cardozo L, Wagg A, Wein A, editors(s). Incontinence: 6th International Consultation on Incontinence. Bristol (UK): ICI-ICS. International Continence Society, 2017:1855-993. [ISBN: 978-0-9569607-3-3]
Maher 2019
-
- Maher CF, Baessler KK, Barber MD, Cheong C, Consten EC, Cooper KG, et al. Surgical management of pelvic organ prolapse. Climacteric 2019;22(3):229-35. - PubMed
Mamik 2014
-
- Mamik MM, Rogers RG, Qualls CR, Morrow JD. The minimum important difference for the pelvic organ prolapse-urinary incontinence sexual function questionnaire. International Urogynecology Journal 2014;25(10):1321-6. - PubMed
Mantel 1959
-
- Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. Journal of the National Cancer Institute 1959;22(4):719-48. - PubMed
Mowat 2018
Naumova 2018
NICE 2019
-
- National Institute for Health and Care Excellence (NICE). Urinary incontinence and pelvic organ prolapse in women: management NICE guideline [NG123]. Published: 2 April 2019; last updated: 24 June 2019. Available at nice.org.uk/guidance/ng123 (accessed 26 June 2022).
North American Menopause Society 2007
-
- North American Menopause Society. The role of local vaginal estrogen for treatment of vaginal atrophy in postmenopausal women: 2007 position statement of The North American Menopause Society. Menopause 2007;14(3 Pt 1):355-369. - PubMed
Olsen 1997
-
- Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstetrics and Gynecology 1997;89(4):501-6. - PubMed
Price 2006
-
- Price N, Jackson SR, Avery K, Brookes ST, Abrams P. Development and psychometric evaluation of the ICIQ vaginal symptoms questionnaire: the ICIQ-VS. BJOG 2006;113(6):700-12. - PubMed
Pruijssers 2021
-
- Pruijssers B, Vaart L, Milani F, Roovers JP, Vollebregt A, Vaart H. Minimal clinically important difference (MCID) for the pelvic organ prolapse-urinary incontinence sexual function questionnaire – IUGA revised (PISQ-IR). Journal of Sexual Medicine 2021;18(7):1265-70. - PubMed
Radley 2006
-
- Radley SC, Jones GL, Tanguy EA, Stevens VG, Nelson C, Mathers NJ. Computer interviewing in urogynaecology: concept, development and psychometric testing of an electronic pelvic floor assessment questionnaire in primary and secondary care. BJOG 2006;113(2):231-8. - PubMed
Rahn 2015
-
- Rahn DD, Ward RM, Sanses TV, Carberry C, Mamik MM, Meriwether KV, et al, on behalf of the Society of Gynecologic Surgeons Systematic Review Group. Vaginal estrogen use in postmenopausal women with pelvic floor disorders: systematic review and practice guidelines. International Urogynecology Journal 2015;26(1):3-13. - PubMed
Review Manager 2020 [Computer program]
-
- Review Manager 5 (RevMan 5). Version 5.4.1. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Salvatore 2017
-
- Salvatore S, Rademakers K, DeLancey J, Igawa Y, Koelbl H, Laterza RM, et al. Pathophysiology of urinary incontinence, faecal incontinence and pelvic organ prolapse. In: Abrams P, Cardozo L, Wagg A, Wein A, editors(s). Incontinence: 6th International Consultation on Incontinence. Bristol (UK): ICI-ICS. International Continence Society, 2017:361-496. [ISBN: 978-0-9569607-3-3]
Schünemann 2021
-
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing 'Summary of findings' tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Sipilä 2001
-
- Sipilä S, Taaffe DR, Cheng S, Puolakka J, Toivanen J, Suominen H. Effects of hormone replacement therapy and high-impact physical exercise on skeletal muscle in post-menopausal women: a randomized placebo-controlled study. Clinical Science 2001;101:147-157. - PubMed
Srikrishna 2010
-
- Srikrishna S, Robinson D, Cardozo L. Validation of the patient global impression of improvement (PGI-I) for urogenital prolapse. International Urogynecology Journal 2010;21(5):523-8. - PubMed
Sterne 2011
-
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011;343:d4002. - PubMed
Stevenson 2009
-
- Stevenson JC. Type and route of estrogen administration. Climacteric 2009;12(Suppl 1):86-90. - PubMed
Subramanian 2009
-
- Subramanian D, Szwarcensztein K, Mauskopf JA, Slack MC. Rate, type, and cost of pelvic organ prolapse surgery in Germany, France, and England. European Journal of Obstetrics, Gynecology and Reproductive Biology 2009;144(2):177-81. - PubMed
Ulrich 2014
-
- Ulrich D, Guzman Rojas R, Dietz HP, Mann K, Trutnovsky G. Use of a visual analog scale for evaluation of bother from pelvic organ prolapse. Ultrasound in Obstetrics and Gynecology 2014;43(6):693-7. - PubMed
Vergeldt 2015
Verghese 2020
-
- Verghese TS, Middleton L, Cheed V, Leighton L, Daniels J, Latthe PM. Randomised controlled trial to investigate the effectiveness of local oestrogen treatment in postmenopausal women undergoing pelvic organ prolapse surgery (LOTUS): a pilot study to assess feasibility of a large multicentre trial. BMJ Open 2020;10(9):e025141. - PMC - PubMed
Vodegel 2021
-
- Vodegel EV, Zwolsman SE, Vollebregt A, Duijnhoven RG, Bosmans JE, Speksnijder L, et al. Cost-effectiveness of perioperative vaginally administered estrogen in postmenopausal women undergoing prolapse surgery (EVA trial): study protocol for a multicenter double-blind randomized placebo-controlled trial. BMC Women's Health 2021;21:439. - PMC - PubMed
Weber 2015
Whiteside 2005
-
- Whiteside JL, Barber MD, Paraiso MF, Walters MD. Vaginal rugae: measurement and significance. Climacteric 2005;8(1):71-5. - PubMed
References to other published versions of this review
Taithongchai 2021
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

