Response Evaluation Criteria in PSMA PET/CT (RECIP 1.0) in Metastatic Castration-resistant Prostate Cancer
- PMID: 37432081
- PMCID: PMC10374938
- DOI: 10.1148/radiol.222148
Response Evaluation Criteria in PSMA PET/CT (RECIP 1.0) in Metastatic Castration-resistant Prostate Cancer
Abstract
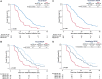
Background Response Evaluation Criteria in Prostate-specific Membrane Antigen (PSMA) PET/CT (RECIP 1.0) initially integrated software-based quantitative assessment of PSMA-positive total tumor volume (TTV). Clinical implementation of such software is not expected soon, limiting the use of RECIP in practice. Purpose To assess the agreement of RECIP determined using tumor segmentation software (quantitative RECIP) with RECIP determined by qualitative reads by nuclear medicine physicians (visual RECIP) for response evaluation in metastatic castration-resistant prostate cancer. Materials and Methods This multicenter retrospective study at three academic centers included men who received lutetium 177 (177Lu) PSMA treatment between December 2014 and July 2019. PSMA PET/CT images at baseline and 12 weeks were assessed qualitatively by five readers for changes in TTV and for new lesions. Quantitative changes in TTV were also measured using tumor segmentation software. The status of new lesions was combined with qualitative changes in TTV to determine visual RECIP and with quantitative changes in TTV to determine quantitative RECIP. The primary outcomes were the agreement between visual and quantitative RECIP and the interreader reliability of visual RECIP according to the Fleiss κ. The secondary outcome was the association of visual RECIP with overall survival according to Cox regression. Results A total of 124 men (median age, 73 years [IQR, 67-76 years]) were included. Forty (32%) and 84 (68%) men had quantitative RECIP progressive disease (PD) and non-PD, respectively. Agreement between visual versus quantitative RECIP was excellent (κ = 0.89; 118 of 124 men [95%]). Agreement among readers in classifying visual RECIP PD versus non-PD was excellent (κ = 0.81; 103 of 124 men [83%]). RECIP PD was associated with significantly shorter overall survival compared with non-PD (hazard ratio, 2.6 [95% CI: 1.7, 3.8]; P < .001). Conclusion Qualitatively assessed RECIP demonstrated excellent agreement with quantitative RECIP and excellent interreader reliability and can be readily implemented in clinical practice for response evaluation in men with metastatic castration-resistant prostate cancer undergoing 177Lu-PSMA therapy. © RSNA, 2023 Supplemental material is available for this article.
Conflict of interest statement
Figures





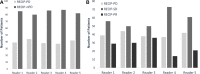
References
-
- Hofman MS , Lawrentschuk N , Francis RJ , et al. . Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study . Lancet 2020. ; 395 ( 10231 ): 1208 – 1216 . - PubMed
-
- Gafita A , Rauscher I , Fendler WP , et al. . Measuring response in metastatic castration-resistant prostate cancer using PSMA PET/CT: comparison of RECIST 1.1, aPCWG3, aPERCIST, PPP, and RECIP 1.0 criteria . Eur J Nucl Med Mol Imaging 2022. ; 49 ( 12 ): 4271 – 4281 . - PubMed
-
- Gafita A , Calais J , Grogan TR , et al. . Nomograms to predict outcomes after 177Lu-PSMA therapy in men with metastatic castration-resistant prostate cancer: an international, multicentre, retrospective study . Lancet Oncol 2021. ; 22 ( 8 ): 1115 – 1125 . - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

