Prevalence of KRAS G12C Mutation and Co-mutations and Associated Clinical Outcomes in Patients With Colorectal Cancer: A Systematic Literature Review
- PMID: 37432264
- PMCID: PMC10628573
- DOI: 10.1093/oncolo/oyad138
Prevalence of KRAS G12C Mutation and Co-mutations and Associated Clinical Outcomes in Patients With Colorectal Cancer: A Systematic Literature Review
Abstract
Purpose: A systematic literature review was conducted to estimate the global prevalence of Kirsten rat sarcoma virus gene (KRAS) mutations, with an emphasis on the clinically significant KRAS G12C mutation, and to estimate the prognostic significance of these mutations in patients with colorectal cancer (CRC).
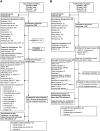
Design: Relevant English-language publications in the Embase, MEDLINE, and the Cochrane Library databases (from 2009 to 2021) and congress presentations (from 2016 to 2021) were reviewed. Eligible studies were those that reported the prevalence and clinical outcomes of the KRAS G12C mutation in patients with CRC.
Results: A total of 137 studies (interventional [n = 8], post hoc analyses of randomized clinical trials [n = 6], observational [n = 122], and longitudinal [n =1]) were reviewed. Sixty-eight studies reported the prevalence of KRAS mutations (KRASm) in 42 810 patients with CRC. The median global prevalence of KRASm was 38% (range, 13.3%-58.9%) and that of the KRAS G12C mutation (KRAS G12C) 3.1% (range, 0.7%-14%). Available evidence suggests that KRASm are possibly more common in tumors that develop on the right side of the colon. Limited evidence suggests a lower objective response rate and inferior disease-free/relapse-free survival in patients with KRAS G12C compared with patients with KRASwt or other KRASm.
Conclusion: Our analysis reveals that KRAS G12C is prevalent in 3% of patients with CRC. Available evidence suggests a poor prognosis for patients with KRAS G12C. Right-sided tumors were more likely to harbor KRASm; however, their role in determining clinical outcomes needs to be investigated further.
Keywords: KRAS G12C; KRASm; colorectal cancer; global; prevalence; prognosis; systematic literature review.
© The Author(s) 2023. Published by Oxford University Press.
Conflict of interest statement
John Strickler reports grants or contracts to research institution from AstraZeneca, Bayer, Seagen, Amgen Inc., Daiichi-Sankyo, Nektar, Abbvie, Erasca, and Gossemer Bio, AStar D3, Curegenix, Sanofi, Roche/ Genentech, and Silverback Therapeutics, consulting fees from Abbvie, Amgen Inc., AstraZeneca, Bayer, Inivata, Natera, GlaxoSmithKline(GSK), Mereo Biopharma, Pionyr Immunotherapeutics, Seagen, Pfizer, Silverback Therapeutics, and Viatris, support for attending meetings and/or travel from Seagen, and participation on a Data Safety Monitoring Board or Advisory Board for Abbvie and Pionyr Immunotherapeutics. Takayuki Yoshino, reports research funding from Ono Pharmaceutical Co., Ltd., Sanofi K.K., Daiichi Sankyo Co., Ltd., PAREXEL International Inc., Pfizer Japan Inc., Taiho Pharmaceutical Co., Ltd., MSD K.K., Amgen K.K., Genomedia Inc., Sysmex Corporation, Chugai Pharmaceutical Co., Ltd., and Nippon Boehringer Ingelheim Co., Ltd., and honoraria from Taiho Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Ltd., Eli Lilly Japan K.K., Merck Biopharma Co., Ltd., Bayer Yakuhin, Ltd., Ono Pharmaceutical Co., Ltd., and MSD K.K. Kendall Stevinson was employed by Amgen Inc. at the time of conduct of the study and has stock ownership in Amgen Inc., is currently employed by Iovance Biotherapeutics, reports research funding from Amgen Inc. and Iovance Biotherapeutics, and travel, accommodation, and expense reimbursement from Iovance Biotherapeutics. Christian Stefan Eichinger is an employee of Oxford Pharmagenesis which received funding for the conduct of this systematic literature review. Christina Giannopoulou is an employee of Amgen (Europe) and reports stock ownership in Amgen (Europe). Marko Rehn, is an employee of Amgen Inc. and reports stock ownership in Amgen Inc.. Dominik Paul Modest reports consultancy (including expert testimony) for Amgen Inc., Servier, AstraZeneca, Merck Sharp & Dohme, Bristol-Myers Squibb, PierreFabre, Sanofi, Lilly, Merck, Cureteq, Onkowissen.de, G1, Incyte, Taiho, Takeda, and COR2ED, research funding to institution from Amgen Inc., and Servier, honoraria from Amgen, Servier, AstraZeneca, Merck Sharp & Dohme, Bristol-Myers Squibb, PierreFabre, Sanofi, Lilly, Merck, Cureteq, Onkowissen.de, G1, Incyte, Taiho, Takeda, and COR2ED.
Figures

References
-
- World Health Organization. Fact sheets: cancer. 2021. Accessed October 20, 2021.https://www.who.int/news-room/fact-sheets/detail/cancer.
-
- Cancer.Net. Colorectal Cancer: Statistics. 2021. Accessed October 05, 2021.https://www.cancer.net/cancer-types/colorectal-cancer/statistics).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

