Concomitant Proton Pump Inhibitor Use With Pembrolizumab Monotherapy vs Immune Checkpoint Inhibitor Plus Chemotherapy in Patients With Non-Small Cell Lung Cancer
- PMID: 37432682
- PMCID: PMC10336622
- DOI: 10.1001/jamanetworkopen.2023.22915
Concomitant Proton Pump Inhibitor Use With Pembrolizumab Monotherapy vs Immune Checkpoint Inhibitor Plus Chemotherapy in Patients With Non-Small Cell Lung Cancer
Abstract
Importance: Immune checkpoint inhibitor (ICI) monotherapy with pembrolizumab and ICI plus chemotherapy have been approved as first-line treatments for non-small cell lung cancer (NSCLC) for patients with a programmed cell death ligand-1 (PD-L1) tumor proportion score (TPS) of 50% or more, but the choice between these 2 therapeutic options is unclear.
Objective: To clarify the association of a history of concurrent medication use with treatment outcomes for ICIs with or without chemotherapy in patients with NSCLC with a high PD-L1 TPS and to determine whether these clinical histories are biomarkers for appropriate treatment selection.
Design, setting, and participants: This retrospective, multicenter cohort study at 13 hospitals in Japan included patients with advanced NSCLC with a PD-L1 TPS of 50% or more who had received pembrolizumab ICI monotherapy or ICI plus chemotherapy as the initial treatment between March 2017 and December 2020. The median (IQR) follow-up duration was 18.5 (9.2-31.2) months. Data were analyzed from April 2022 through May 2023.
Exposure: ICI monotherapy with pembrolizumab or ICI plus chemotherapy as first-line treatment.
Main outcomes and measures: The primary analysis was the association of treatment outcomes with baseline patient characteristics, including concomitant drug history, after propensity score matching. Cox proportional hazard models were used to determine the associations of patient characteristics with survival outcomes. Logistic regression analysis was used to determine the association of concomitant medication history with treatment outcomes and other patient characteristics.
Results: A total of 425 patients with NSCLC were enrolled in the study including 271 patients (median [range] age, 72 [43-90] years; 215 [79%] men) who were treated with pembrolizumab monotherapy as the first-line treatment and 154 patients (median [range] age, 69 [36-86] years; 121 [79%] men) who were treated with ICI plus chemotherapy as the first-line treatment. In multivariable analysis, a history of proton pump inhibitor (PPI) use was independently associated with shorter progression-free survival (PFS) in the pembrolizumab monotherapy group (hazard ratio [HR], 1.38; 95% CI, 1.00-1.91; P = .048), but not in the ICI plus chemotherapy group. In patients with a PPI history, both the median (IQR) PFS (19.3 [9.0 to not reached] months vs 5.7 [2.4 to 15.2] months; HR, 0.38; 95% CI, 0.20-0.72; P = .002) and the median (IQR) overall survival (not reached [9.0 months to not reached) vs 18.4 [10.5 to 50.0] months; HR, 0.43; 95% CI, 0.20-0.92; P = .03) were significantly longer in the ICI plus chemotherapy group than in the pembrolizumab monotherapy group. In patients without a history of PPI use, both the median (IQR) PFS (18.8 months [6.6 months to not reached] vs 10.6 months [2.7 months to not reached]; HR, 0.81; 95% CI, 0.56-1.17; P = .26) and the median (IQR) overall survival (not reached [12.6 months to not reached] vs 29.9 [13.3 to 54.3] months, HR, 0.75; 95% CI, 0.48-1.18; P = .21) did not differ between groups.
Conclusions and relevance: This cohort study found that a history of PPI use could be an important clinical factor in treatment decision-making for patients with NSCLC with a PD-L1 TPS of 50% or more.
Conflict of interest statement
Figures
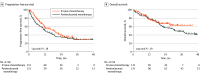
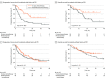
Comment in
-
Is Immunotherapy With Concomitant Proton Pump Inhibitor Use a Viable Combination?JAMA Netw Open. 2023 Jul 3;6(7):e2322922. doi: 10.1001/jamanetworkopen.2023.22922. JAMA Netw Open. 2023. PMID: 37432691 No abstract available.
References
-
- Rittmeyer A, Barlesi F, Waterkamp D, et al. ; OAK Study Group . Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255-265. doi:10.1016/S0140-6736(16)32517-X - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

