Baseline Features and Reasons for Nonparticipation in the Colonoscopy Versus Fecal Immunochemical Test in Reducing Mortality From Colorectal Cancer (CONFIRM) Study, a Colorectal Cancer Screening Trial
- PMID: 37432690
- PMCID: PMC10336619
- DOI: 10.1001/jamanetworkopen.2023.21730
Baseline Features and Reasons for Nonparticipation in the Colonoscopy Versus Fecal Immunochemical Test in Reducing Mortality From Colorectal Cancer (CONFIRM) Study, a Colorectal Cancer Screening Trial
Erratum in
-
Error in Byline.JAMA Netw Open. 2023 Aug 1;6(8):e2330304. doi: 10.1001/jamanetworkopen.2023.30304. JAMA Netw Open. 2023. PMID: 37566425 Free PMC article. No abstract available.
Abstract
Importance: The Colonoscopy Versus Fecal Immunochemical Test in Reducing Mortality From Colorectal Cancer (CONFIRM) randomized clinical trial sought to recruit 50 000 adults into a study comparing colorectal cancer (CRC) mortality outcomes after randomization to either an annual fecal immunochemical test (FIT) or colonoscopy.
Objective: To (1) describe study participant characteristics and (2) examine who declined participation because of a preference for colonoscopy or stool testing (ie, fecal occult blood test [FOBT]/FIT) and assess that preference's association with geographic and temporal factors.
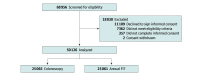
Design, setting, and participants: This cross-sectional study within CONFIRM, which completed enrollment through 46 Department of Veterans Affairs medical centers between May 22, 2012, and December 1, 2017, with follow-up planned through 2028, comprised veterans aged 50 to 75 years with an average CRC risk and due for screening. Data were analyzed between March 7 and December 5, 2022.
Exposure: Case report forms were used to capture enrolled participant data and reasons for declining participation among otherwise eligible individuals.
Main outcomes and measures: Descriptive statistics were used to characterize the cohort overall and by intervention. Among individuals declining participation, logistic regression was used to compare preference for FOBT/FIT or colonoscopy by recruitment region and year.
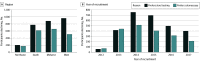
Results: A total of 50 126 participants were recruited (mean [SD] age, 59.1 [6.9] years; 46 618 [93.0%] male and 3508 [7.0%] female). The cohort was racially and ethnically diverse, with 748 (1.5%) identifying as Asian, 12 021 (24.0%) as Black, 415 (0.8%) as Native American or Alaska Native, 34 629 (69.1%) as White, and 1877 (3.7%) as other race, including multiracial; and 5734 (11.4%) as having Hispanic ethnicity. Of the 11 109 eligible individuals who declined participation (18.0%), 4824 (43.4%) declined due to a stated preference for a specific screening test, with FOBT/FIT being the most preferred method (2820 [58.5%]) vs colonoscopy (1958 [40.6%]; P < .001) or other screening tests (46 [1.0%] P < .001). Preference for FOBT/FIT was strongest in the West (963 of 1472 [65.4%]) and modest elsewhere, ranging from 199 of 371 (53.6%) in the Northeast to 884 of 1543 (57.3%) in the Midwest (P = .001). Adjusting for region, the preference for FOBT/FIT increased by 19% per recruitment year (odds ratio, 1.19; 95% CI, 1.14-1.25).
Conclusions and relevance: In this cross-sectional analysis of veterans choosing nonenrollment in the CONFIRM study, those who declined participation more often preferred FOBT or FIT over colonoscopy. This preference increased over time and was strongest in the western US and may provide insight into trends in CRC screening preferences.
Conflict of interest statement
Figures