Targeting Anaerobic Respiration in Pseudomonas aeruginosa with Chlorate Improves Healing of Chronic Wounds
- PMID: 37432895
- PMCID: PMC10659023
- DOI: 10.1089/wound.2023.0036
Targeting Anaerobic Respiration in Pseudomonas aeruginosa with Chlorate Improves Healing of Chronic Wounds
Abstract
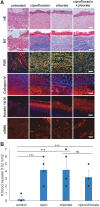
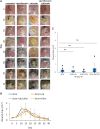
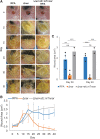
Objective: Pseudomonas aeruginosa is an opportunistic pathogen that can establish chronic infections and form biofilm in wounds. Because the wound environment is largely devoid of oxygen, P. aeruginosa may rely on anaerobic metabolism, such as nitrate respiration, to survive in wounds. While nitrate reductase (Nar) typically reduces nitrate to nitrite, it can also reduce chlorate to chlorite, which is a toxic oxidizing agent. Therefore, chlorate can act as a prodrug to specifically eradicate hypoxic/anoxic, nitrate-respiring P. aeruginosa populations, which are often tolerant to conventional antibiotic treatments. Approach: Using a diabetic mouse model for chronic wounds, we tested the role that anaerobic nitrate respiration plays in supporting chronic P. aeruginosa infections. Results: P. aeruginosa forms biofilm deep within the wound where the environment is anoxic. Daily treatment of P. aeruginosa-infected wounds with chlorate supported wound healing. Chlorate treatment was as effective as a treatment with ciprofloxacin (a conventional antibiotic that targets both oxic and hypoxic/anoxic P. aeruginosa populations). Chlorate-treated wounds showed markers of good-quality wound healing, including well-formed granulation tissue, reepithelialization and microvessel development. Loss- and gain-of-function experiments showed that P. aeruginosa requires nitrate respiration to establish a chronic wound infection and form biofilms. Innovation: We show that the small molecule chlorate, kills the opportunistic pathogen, P. aeruginosa, by targeting a form of anaerobic metabolism called nitrate respiration. Conclusion: Chlorate holds promise as a treatment to combat diverse bacterial infections where oxygen is limiting and/or where pathogens grow as biofilms because many other pathogens possess Nar and survive using anaerobic metabolism.
Keywords: MiPACT-HCR; Pseudomonas aeruginosa; anaerobic respiration; antibiotics; chlorate; chronic wounds.
Conflict of interest statement
The authors state no competing financial interests. The content of this article was expressly written by the authors listed. No ghostwriters were used to write this article.
Figures





Similar articles
-
Chlorate Specifically Targets Oxidant-Starved, Antibiotic-Tolerant Populations of Pseudomonas aeruginosa Biofilms.mBio. 2018 Sep 25;9(5):e01400-18. doi: 10.1128/mBio.01400-18. mBio. 2018. PMID: 30254119 Free PMC article.
-
Mechanisms of chlorate toxicity and resistance in Pseudomonas aeruginosa.Mol Microbiol. 2022 Oct;118(4):321-335. doi: 10.1111/mmi.14972. Epub 2022 Aug 15. Mol Microbiol. 2022. PMID: 36271736 Free PMC article.
-
Pseudomonas aeruginosa biofilms perturb wound resolution and antibiotic tolerance in diabetic mice.Med Microbiol Immunol. 2013 Apr;202(2):131-41. doi: 10.1007/s00430-012-0277-7. Epub 2012 Sep 25. Med Microbiol Immunol. 2013. PMID: 23007678 Free PMC article.
-
Anaerobic bacteria in chronic wounds: Roles in disease, infection and treatment failure.Wound Repair Regen. 2024 Nov-Dec;32(6):840-857. doi: 10.1111/wrr.13208. Epub 2024 Aug 12. Wound Repair Regen. 2024. PMID: 39129662 Review.
-
Microenvironmental characteristics and physiology of biofilms in chronic infections of CF patients are strongly affected by the host immune response.APMIS. 2017 Apr;125(4):276-288. doi: 10.1111/apm.12668. APMIS. 2017. PMID: 28407427 Review.
Cited by
-
Pseudomonas aeruginosa Activates Quorum Sensing, Antioxidant Enzymes and Type VI Secretion in Response to Oxidative Stress to Initiate Biofilm Formation and Wound Chronicity.Antioxidants (Basel). 2024 May 27;13(6):655. doi: 10.3390/antiox13060655. Antioxidants (Basel). 2024. PMID: 38929094 Free PMC article.
-
mSphere of Influence: The power of polymicrobial partnerships in chronic infection research.mSphere. 2024 Sep 25;9(9):e0043424. doi: 10.1128/msphere.00434-24. Epub 2024 Aug 20. mSphere. 2024. PMID: 39162472 Free PMC article.
-
The Impact of the Skin Microbiome and Oxidative Stress on the Initiation and Development of Cutaneous Chronic Wounds.Antioxidants (Basel). 2025 Jun 4;14(6):682. doi: 10.3390/antiox14060682. Antioxidants (Basel). 2025. PMID: 40563316 Free PMC article. Review.

