Assessing a biomarker's ability to reduce invasive procedures in patients with benign lung nodules: Results from the ORACLE study
- PMID: 37432960
- PMCID: PMC10335667
- DOI: 10.1371/journal.pone.0287409
Assessing a biomarker's ability to reduce invasive procedures in patients with benign lung nodules: Results from the ORACLE study
Abstract
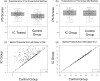
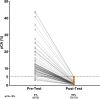
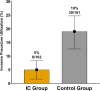
A blood-based integrated classifier (IC) has been clinically validated to improve accuracy in assessing probability of cancer risk (pCA) for pulmonary nodules (PN). This study evaluated the clinical utility of this biomarker for its ability to reduce invasive procedures in patients with pre-test pCA ≤ 50%. This was a propensity score matching (PSM) cohort study comparing patients in the ORACLE prospective, multicenter, observational registry to control patients treated with usual care. This study enrolled patients meeting the intended use criteria for IC testing: pCA ≤ 50%, age ≥40 years, nodule diameter 8-30 mm, and no history of lung cancer and/or active cancer (except for non-melanomatous skin cancer) within 5 years. The primary aim of this study was to evaluate invasive procedure use on benign PNs of registry patients as compared to control patients. A total of 280 IC tested, and 278 control patients met eligibility and analysis criteria and 197 were in each group after PSM (IC and control groups). Patients in the IC group were 74% less likely to undergo an invasive procedure as compared to the control group (absolute difference 14%, p <0.001) indicating that for every 7 patients tested, one unnecessary invasive procedure was avoided. Invasive procedure reduction corresponded to a reduction in risk classification, with 71 patients (36%) in the IC group classified as low risk (pCA < 5%). The proportion of IC group patients with malignant PNs sent to surveillance were not statistically different than the control group, 7.5% vs 3.5% for the IC vs. control groups, respectively (absolute difference 3.91%, p 0.075). The IC for patients with a newly discovered PN has demonstrated valuable clinical utility in a real-world setting. Use of this biomarker can change physicians' practice and reduce invasive procedures in patients with benign pulmonary nodules. Trial registration: Clinical trial registration: ClinicalTrials.gov NCT03766958.
Copyright: © 2023 Pritchett et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have reported the following: M.A.P. discloses immediate family member employment (Medtronic, Philips), honoraria (Medtronic, Philips, Astra Zeneca), consulting (Medtronic, Philips, Intuitive, Johnson & Johnson, Pfizer, Noah Medical), research funding (Medtronic, Philips, Biodesix, Inc.), speaker’s bureau (Johnson & Johnson, United Therapeutics, Biodesix, Inc.), and travel/accommodations/expenses (Intuitive, Johnson & Johnson, Astra Zeneca, Pfizer, Noah Medical). B S. reports nothing to disclose. M.R. B. discloses consulting (Medtronic). J.S.K. discloses consulting/advisory (Ambu, Biodesix, Inc., Boston Scientific, Cook, Intuitive, Level Ex, Medtronic, Pulmonx), research funding (Lung Therapeutics, PrognomiQ), honoraria (Pinnacle Biologics), speaker’s bureau (Biodesix, Inc., Veracyte), stock ownership (Doximity), and travel/accommodations/expenses (Auris, Pinnacle Biologics). T.P. reports employment (Biodesix, Inc.). S.C.S. reports employment and leadership (Biodesix, Inc.). There are no patents or products in development associated with this research to declare. The IC (Nodify XL2) is marketed by Biodesix, Inc. in Boulder, Colorado. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures
References
-
- Gould MK, Donington J, Lynch WR, Mazzone PJ, Midthun DE, Naidich DP, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e93S–e120S. Epub 2013/05/10. doi: 10.1378/chest.12-2351 ; PubMed Central PMCID: PMC3749714. - DOI - PMC - PubMed
-
- Swensen SJ, Silverstein MD, Ilstrup DM, Schleck CD, Edell ES. The probability of malignancy in solitary pulmonary nodules. Application to small radiologically indeterminate nodules. Arch Intern Med. 1997;157(8):849–55. Epub 1997/04/28. . - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

