How enhancers regulate wavelike gene expression patterns
- PMID: 37432987
- PMCID: PMC10368423
- DOI: 10.7554/eLife.84969
How enhancers regulate wavelike gene expression patterns
Abstract
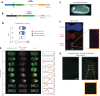
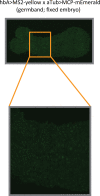
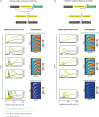
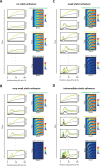
A key problem in development is to understand how genes turn on or off at the right place and right time during embryogenesis. Such decisions are made by non-coding sequences called 'enhancers.' Much of our models of how enhancers work rely on the assumption that genes are activated de novo as stable domains across embryonic tissues. Such a view has been strengthened by the intensive landmark studies of the early patterning of the anterior-posterior (AP) axis of the Drosophila embryo, where indeed gene expression domains seem to arise more or less stably. However, careful analysis of gene expression patterns in other model systems (including the AP patterning in vertebrates and short-germ insects like the beetle Tribolium castaneum) painted a different, very dynamic view of gene regulation, where genes are oftentimes expressed in a wavelike fashion. How such gene expression waves are mediated at the enhancer level is so far unclear. Here, we establish the AP patterning of the short-germ beetle Tribolium as a model system to study dynamic and temporal pattern formation at the enhancer level. To that end, we established an enhancer prediction system in Tribolium based on time- and tissue-specific ATAC-seq and an enhancer live reporter system based on MS2 tagging. Using this experimental framework, we discovered several Tribolium enhancers, and assessed the spatiotemporal activities of some of them in live embryos. We found our data consistent with a model in which the timing of gene expression during embryonic pattern formation is mediated by a balancing act between enhancers that induce rapid changes in gene expression patterns (that we call 'dynamic enhancers') and enhancers that stabilize gene expression patterns (that we call 'static enhancers'). However, more data is needed for a strong support for this or any other alternative models.
Keywords: clock; computational biology; developmental biology; enhancers; pattern formation; systems biology; temporal patterning; tribolium; waves.
© 2023, Mau et al.
Conflict of interest statement
CM, HR, FS, BS, TR, RP, ES, LT, EE No competing interests declared
Figures

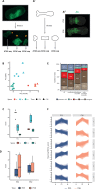
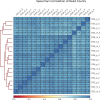

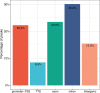
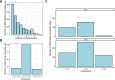



Update of
References
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

