Effect of Malaria Infection on Epstein-Barr Virus Persistence in Kenyan Children
- PMID: 37433031
- PMCID: PMC10786253
- DOI: 10.1093/infdis/jiad264
Effect of Malaria Infection on Epstein-Barr Virus Persistence in Kenyan Children
Abstract
Background: The 2 cofactors in the etiology of Burkitt lymphoma (BL) are Epstein-Barr virus (EBV) and repeated Plasmodium falciparum malaria infections. This study evaluated EBV loads in mucosal and systemic compartments of children with malaria and controls. Age was analyzed as a covariate because immunity to malaria in endemic regions is age dependent.
Methods: Children (2-10 years) with clinical malaria from Western Kenya and community controls without malaria were enrolled. Saliva and blood samples were collected, EBV viral load was assessed by quantitative polymerase chain reaction, and EpiTYPER MassARRAY was used to assess methylation of 3 different EBV genes.
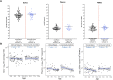
Results: Regardless of the compartment, we detected EBV more frequently in malaria cases compared to controls, although the difference was not significant. When EBV was detected, there were no differences in viral load between cases and controls. However, EBV methylation was significantly lower in the malaria group compared to controls in both plasma and saliva (P < .05), indicating increased EBV lytic replication. In younger children before development of immunity to malaria, there was a significant effect of malaria on EBV load in peripheral blood mononuclear cells (P = .04).
Conclusions: These data suggest that malaria can directly modulate EBV persistence in children, increasing their risk for BL.
Keywords: Burkitt lymphoma; EBV methylation; EpiTYPER; Epstein-Barr virus; malaria.
© The Author(s) 2023. Published by Oxford University Press on behalf of Infectious Diseases Society of America. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
Potential conflicts of interest. All authors: No reported conflicts of interest.
Figures

References
-
- Thorley-Lawson DA. Epstein-Barr virus: exploiting the immune system. Nat Rev Immunol 2001; 1:75–82. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

