Structure Property Relationship of Micellar Waterborne Poly(Urethane-Urea): Tunable Mechanical Properties and Controlled Release Profiles with Amphiphilic Triblock Copolymers
- PMID: 37433143
- PMCID: PMC10373496
- DOI: 10.1021/acs.langmuir.3c00921
Structure Property Relationship of Micellar Waterborne Poly(Urethane-Urea): Tunable Mechanical Properties and Controlled Release Profiles with Amphiphilic Triblock Copolymers
Abstract
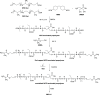
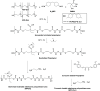
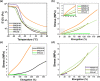
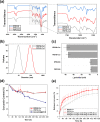
Waterborne polyurethane (WPU) has attracted significant interest as a promising alternative to solvent-based polyurethane (SPU) due to its positive impact on safety and sustainability. However, significant limitations of WPU, such as its weaker mechanical strength, limit its ability to replace SPU. Triblock amphiphilic diols are promising materials to enhance the performance of WPU due to their well-defined hydrophobic-hydrophilic structures. Yet, our understanding of the relationship between the hydrophobic-hydrophilic arrangements of triblock amphiphilic diols and the physical properties of WPU remains limited. In this study, we show that by controlling the micellar structure of WPU in aqueous solution via the introduction of triblock amphiphilic diols, the postcuring efficiency and the resulting mechanical strength of WPU can be significantly enhanced. Small-angle neutron scattering confirmed the microstructure and spatial distribution of hydrophilic and hydrophobic segments in the engineered WPU micelles. In addition, we show that the control of the WPU micellar structure through triblock amphiphilic diols renders WPU attractive in the applications of controlled release, such as drug delivery. Here, curcumin was used as a model hydrophobic drug, and the drug release behavior from WPU-micellar-based drug delivery systems was characterized. It was found that curcumin-loaded WPU drug delivery systems were highly biocompatible and exhibited antibacterial properties in vitro. Furthermore, the sustained release profile of the drug was found to be dependent on the structure of the triblock amphiphilic diols, suggesting the possibility of controlling the drug release profile via the selection of triblock amphiphilic diols. This work shows that by shedding light on the structure-property relationship of triblock amphiphilic diol-containing WPU micelles, we may enhance the applicability of WPU systems and move closer to realizing their promising potential in real-life applications.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
Self-assembled micelles of biodegradable triblock copolymers based on poly(ethyl ethylene phosphate) and poly(-caprolactone) as drug carriers.Biomacromolecules. 2008 Jan;9(1):388-95. doi: 10.1021/bm700732g. Epub 2007 Dec 15. Biomacromolecules. 2008. PMID: 18081252
-
Fabrication of Conjugated Amphiphilic Triblock Copolymer for Drug Delivery and Fluorescence Cell Imaging.ACS Biomater Sci Eng. 2018 Feb 12;4(2):566-575. doi: 10.1021/acsbiomaterials.7b00991. Epub 2018 Jan 23. ACS Biomater Sci Eng. 2018. PMID: 33418746
-
Thermo-responsive release of curcumin from micelles prepared by self-assembly of amphiphilic P(NIPAAm-co-DMAAm)-b-PLLA-b-P(NIPAAm-co-DMAAm) triblock copolymers.Int J Pharm. 2014 Dec 10;476(1-2):31-40. doi: 10.1016/j.ijpharm.2014.09.029. Epub 2014 Sep 24. Int J Pharm. 2014. PMID: 25260217
-
Advances in Waterborne Polyurethane-Based Biomaterials for Biomedical Applications.Adv Exp Med Biol. 2018;1077:251-283. doi: 10.1007/978-981-13-0947-2_14. Adv Exp Med Biol. 2018. PMID: 30357693 Review.
-
Novel Pentablock Copolymers as Thermosensitive Self-Assembling Micelles for Ocular Drug Delivery.Adv Pharm Bull. 2017 Apr;7(1):11-20. doi: 10.15171/apb.2017.003. Epub 2017 Apr 13. Adv Pharm Bull. 2017. PMID: 28507933 Free PMC article. Review.
Cited by
-
Key Transdermal Patch Using Cannabidiol-Loaded Nanocarriers with Better Pharmacokinetics in vivo.Int J Nanomedicine. 2024 May 16;19:4321-4337. doi: 10.2147/IJN.S455032. eCollection 2024. Int J Nanomedicine. 2024. PMID: 38770103 Free PMC article.
References
-
- Kuo C.-F. J.; Chen J.-B.; Yang C.-P.; Dong M.-Y. Process Developmentof Water-Based Polyurethane with acrylate Terminal Group under Water Vapor Permeability and Water Repellency for Nylon Fabric. Text. Res. J. 2021, 91 (5–6), 570–579. 10.1177/0040517520948187. - DOI
-
- Tian Y.; Huang X.; Cheng Y.; Niu Y.; Ma J.; Zhao Y.; Kou X.; Ke Q. Applications of Adhesives in Textiles: A Review. Eur. Polym. J. 2022, 167, 11108910.1016/j.eurpolymj.2022.111089. - DOI
-
- Li C.; Xiao H.; Wang X.; Zhao T. Development of Green Waterborne UV-Curable Vegetable Oil-Based Urethane acrylate Pigment Prints Adhesive: Preparation and Application. J. Cleaner Prod. 2018, 180, 272–279. 10.1016/j.jclepro.2018.01.193. - DOI
LinkOut - more resources
Full Text Sources

