Ca2+ spark latency and control of intrinsic Ca2+ release dyssynchrony in rat cardiac ventricular muscle cells
- PMID: 37433391
- PMCID: PMC7616665
- DOI: 10.1016/j.yjmcc.2023.07.005
Ca2+ spark latency and control of intrinsic Ca2+ release dyssynchrony in rat cardiac ventricular muscle cells
Abstract
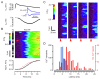
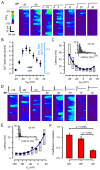
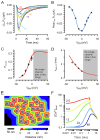
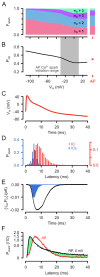
Cardiac excitation-contraction coupling (ECC) depends on Ca2+ release from intracellular stores via ryanodine receptors (RyRs) triggered by L-type Ca2+ channels (LCCs). Uncertain numbers of RyRs and LCCs form 'couplons' whose activation produces Ca2+ sparks, which summate to form a cell-wide Ca2+ transient that switches on contraction. Voltage (Vm) changes during the action potential (AP) and stochasticity in channel gating should create variability in Ca2+ spark timing, but Ca2+ transient wavefronts have remarkable uniformity. To examine how this is achieved, we measured the Vm-dependence of evoked Ca2+ spark probability (Pspark) and latency over a wide voltage range in rat ventricular cells. With depolarising steps, Ca2+ spark latency showed a U-shaped Vm-dependence, while repolarising steps from 50 mV produced Ca2+ spark latencies that increased monotonically with Vm. A computer model based on reported channel gating and geometry reproduced our experimental data and revealed a likely RyR:LCC stoichiometry of ∼ 5:1 for the Ca2+ spark initiating complex (IC). Using the experimental AP waveform, the model revealed a high coupling fidelity (Pcpl ∼ 0.5) between each LCC opening and IC activation. The presence of ∼ 4 ICs per couplon reduced Ca2+ spark latency and increased Pspark to match experimental data. Variability in AP release timing is less than that seen with voltage steps because the AP overshoot and later repolarization decrease Pspark due to effects on LCC flux and LCC deactivation respectively. This work provides a framework for explaining the Vm- and time-dependence of Pspark, and indicates how ion channel dispersion in disease can contribute to dyssynchrony in Ca2+ release.
Keywords: Action potential; Ca(2+) sparks; Cardiac myocytes; Excitation-contraction coupling; Heart; Intracellular Ca(2+).
Copyright © 2023. Published by Elsevier Ltd.
Conflict of interest statement
Declaration of Competing Interest The authors declare no competing interest.
Figures

Similar articles
-
Ryanodine receptor sensitivity governs the stability and synchrony of local calcium release during cardiac excitation-contraction coupling.J Mol Cell Cardiol. 2016 Mar;92:82-92. doi: 10.1016/j.yjmcc.2016.01.024. Epub 2016 Jan 28. J Mol Cell Cardiol. 2016. PMID: 26827896 Free PMC article.
-
A novel mechanism of tandem activation of ryanodine receptors by cytosolic and SR luminal Ca2+ during excitation-contraction coupling in atrial myocytes.J Physiol. 2017 Jun 15;595(12):3835-3845. doi: 10.1113/JP273611. Epub 2017 Feb 1. J Physiol. 2017. PMID: 28028837 Free PMC article.
-
Calcium signaling between sarcolemmal calcium channels and ryanodine receptors in heart cells.Front Biosci. 2002 Sep 1;7:d1867-78. doi: 10.2741/A885. Front Biosci. 2002. PMID: 12161336 Review.
-
Using a Failing Human Ventricular Cardiomyocyte Model to Re-Evaluate Ca2+ Cycling, Voltage Dependence, and Spark Characteristics.Biomolecules. 2024 Oct 28;14(11):1371. doi: 10.3390/biom14111371. Biomolecules. 2024. PMID: 39595549 Free PMC article.
-
[Microscopic mechanism of excitation-contraction coupling in cardiac myocytes].Sheng Li Ke Xue Jin Zhan. 2004 Oct;35(4):294-8. Sheng Li Ke Xue Jin Zhan. 2004. PMID: 15727204 Review. Chinese.
References
-
- Lai FA, Anderson K, Rousseau E, Liu Q-Y, Meissner G. Evidence for a Ca2+ channel within the ryanodine receptor complex from cardiac sarcoplasmic reticulum. Biochem Biophys Res Commun. 1988;151:441–449. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

