Safety and feasibility of platelet transfusion through long catheters in the neonatal intensive care unit: an in vitro study
- PMID: 37433587
- PMCID: PMC10803993
- DOI: 10.1136/archdischild-2023-325632
Safety and feasibility of platelet transfusion through long catheters in the neonatal intensive care unit: an in vitro study
Abstract
Objective: To assess the safety and feasibility of platelet transfusion through small-bore long lines used in the neonatal intensive care unit (NICU), including double-lumen umbilical venous catheters (UVCs) and 24 G and 28 G peripherally inserted central catheters (PICCs).
Design: Prospective in vitro controlled study.
Setting: Blood transfusion service laboratory.
Methods: In vitro platelet transfusions were set up as per NICU practice. Transfusion line pressure was monitored. Post-transfusion swirling, presence of aggregates, pH analysis and automated cell count in vitro activation response by flow cytometry assessing CD62P expression were assessed.
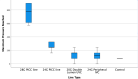
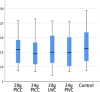
Main outcome measures: All transfusions completed successfully. The rate of infusion was reduced in 5 of 16 transfusions through 28 G lines due to 'pressure high' alarms. There was no difference in swirling values or transfusion aggregate formation, CD62P expression levels, platelet count, platelet distribution width, mean platelet volume, plateletcrit or platelet to large cell ratio across transfusions post-transfusion.
Conclusions: This study showed that in vitro platelet transfusion performed through 24 G and 28 G neonatal PICC lines and double-lumen UVCs is non-inferior to 24 G short cannulas, using outcome measures of platelet clumping, platelet activation and line occlusion. This suggests that where available these lines can be used if necessary for platelet transfusion.
Keywords: Intensive Care Units, Neonatal; Neonatology; Technology.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures

Similar articles
-
Platelet Transfusions in a Multi-Neonatal Intensive Care Unit Health Care Organization Before and After Publication of the PlaNeT-2 Clinical Trial.J Pediatr. 2023 Jun;257:113388. doi: 10.1016/j.jpeds.2023.03.003. Epub 2023 Mar 16. J Pediatr. 2023. PMID: 36933765 Clinical Trial.
-
Platelet transfusion in the neonatal intensive care unit: benefits, risks, alternatives.Neonatology. 2011;100(3):311-8. doi: 10.1159/000329925. Epub 2011 Oct 3. Neonatology. 2011. PMID: 21986337 Review.
-
Blood transfusions using 27 gauge PICC lines: a retrospective clinical study on safety and feasibility.Klin Padiatr. 2014 Jan;226(1):3-7. doi: 10.1055/s-0033-1363244. Epub 2014 Jan 16. Klin Padiatr. 2014. PMID: 24435791
-
Transfusing neonates based on platelet count vs. platelet mass: a randomized feasibility-pilot study.Platelets. 2014;25(7):513-6. doi: 10.3109/09537104.2013.843072. Epub 2013 Nov 13. Platelets. 2014. PMID: 24224920 Clinical Trial.
-
Advances and controversies in neonatal ICU platelet transfusion practice.Adv Pediatr. 2008;55:255-69. doi: 10.1016/j.yapd.2008.07.003. Adv Pediatr. 2008. PMID: 19048733 Review.
References
MeSH terms
LinkOut - more resources
Full Text Sources
