Prevalence and predictors of data and code sharing in the medical and health sciences: systematic review with meta-analysis of individual participant data
- PMID: 37433624
- PMCID: PMC10334349
- DOI: 10.1136/bmj-2023-075767
Prevalence and predictors of data and code sharing in the medical and health sciences: systematic review with meta-analysis of individual participant data
Abstract
Objectives: To synthesise research investigating data and code sharing in medicine and health to establish an accurate representation of the prevalence of sharing, how this frequency has changed over time, and what factors influence availability.
Design: Systematic review with meta-analysis of individual participant data.
Data sources: Ovid Medline, Ovid Embase, and the preprint servers medRxiv, bioRxiv, and MetaArXiv were searched from inception to 1 July 2021. Forward citation searches were also performed on 30 August 2022.
Review methods: Meta-research studies that investigated data or code sharing across a sample of scientific articles presenting original medical and health research were identified. Two authors screened records, assessed the risk of bias, and extracted summary data from study reports when individual participant data could not be retrieved. Key outcomes of interest were the prevalence of statements that declared that data or code were publicly or privately available (declared availability) and the success rates of retrieving these products (actual availability). The associations between data and code availability and several factors (eg, journal policy, type of data, trial design, and human participants) were also examined. A two stage approach to meta-analysis of individual participant data was performed, with proportions and risk ratios pooled with the Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis.
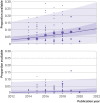
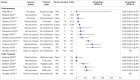
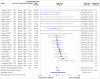
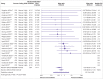
Results: The review included 105 meta-research studies examining 2 121 580 articles across 31 specialties. Eligible studies examined a median of 195 primary articles (interquartile range 113-475), with a median publication year of 2015 (interquartile range 2012-2018). Only eight studies (8%) were classified as having a low risk of bias. Meta-analyses showed a prevalence of declared and actual public data availability of 8% (95% confidence interval 5% to 11%) and 2% (1% to 3%), respectively, between 2016 and 2021. For public code sharing, both the prevalence of declared and actual availability were estimated to be <0.5% since 2016. Meta-regressions indicated that only declared public data sharing prevalence estimates have increased over time. Compliance with mandatory data sharing policies ranged from 0% to 100% across journals and varied by type of data. In contrast, success in privately obtaining data and code from authors historically ranged between 0% and 37% and 0% and 23%, respectively.
Conclusions: The review found that public code sharing was persistently low across medical research. Declarations of data sharing were also low, increasing over time, but did not always correspond to actual sharing of data. The effectiveness of mandatory data sharing policies varied substantially by journal and type of data, a finding that might be informative for policy makers when designing policies and allocating resources to audit compliance.
Systematic review registration: Open Science Framework doi:10.17605/OSF.IO/7SX8U.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at https://www.icmje.org/disclosure-of-interest/ and declare: support from the Australian Research Council during the conduct of this research for the submitted work; some authors had support from research institutions listed in the funding statement; some authors received financial support to attend an international congress to present preliminary results of this project; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Figures





Similar articles
-
How often do cancer researchers make their data and code available and what factors are associated with sharing?BMC Med. 2022 Nov 9;20(1):438. doi: 10.1186/s12916-022-02644-2. BMC Med. 2022. PMID: 36352426 Free PMC article.
-
Rates and predictors of data and code sharing in the medical and health sciences: Protocol for a systematic review and individual participant data meta-analysis.F1000Res. 2021 Jun 22;10:491. doi: 10.12688/f1000research.53874.2. eCollection 2021. F1000Res. 2021. PMID: 34631024 Free PMC article.
-
Beyond the black stump: rapid reviews of health research issues affecting regional, rural and remote Australia.Med J Aust. 2020 Dec;213 Suppl 11:S3-S32.e1. doi: 10.5694/mja2.50881. Med J Aust. 2020. PMID: 33314144
-
Public sector reforms and their impact on the level of corruption: A systematic review.Campbell Syst Rev. 2021 May 24;17(2):e1173. doi: 10.1002/cl2.1173. eCollection 2021 Jun. Campbell Syst Rev. 2021. PMID: 37131927 Free PMC article. Review.
-
The effect of exposure to long working hours on stroke: A systematic review and meta-analysis from the WHO/ILO Joint Estimates of the Work-related Burden of Disease and Injury.Environ Int. 2020 Sep;142:105746. doi: 10.1016/j.envint.2020.105746. Epub 2020 Jun 3. Environ Int. 2020. PMID: 32505015
Cited by
-
Indicators of transparency and data sharing in scientific writing in published randomized controlled trials in orthodontic journals between 2019 and 2023: an empirical study.Eur J Orthod. 2024 Dec 1;46(6):cjae064. doi: 10.1093/ejo/cjae064. Eur J Orthod. 2024. PMID: 39569723 Free PMC article.
-
Building transparency and reproducibility into the practice of pharmacoepidemiology and outcomes research.Am J Epidemiol. 2024 Nov 4;193(11):1625-1631. doi: 10.1093/aje/kwae087. Am J Epidemiol. 2024. PMID: 38794897 Free PMC article.
-
Evaluating the Reproducibility and Verifiability of Nutrition Research: A Case Study of Studies Assessing the Relationship Between Potatoes and Colorectal Cancer.medRxiv [Preprint]. 2024 Dec 2:2024.12.01.24318272. doi: 10.1101/2024.12.01.24318272. medRxiv. 2024. PMID: 39677420 Free PMC article. Preprint.
-
Six solutions for clinical study data sharing in Germany.BMC Med Res Methodol. 2025 May 24;25(1):140. doi: 10.1186/s12874-025-02560-y. BMC Med Res Methodol. 2025. PMID: 40413408 Free PMC article.
-
The State of Data Sharing in Plastic Surgery: An Analysis of Journal Practices and Author Adherence.Plast Reconstr Surg Glob Open. 2025 May 8;13(5):e6761. doi: 10.1097/GOX.0000000000006761. eCollection 2025 May. Plast Reconstr Surg Glob Open. 2025. PMID: 40342988 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
