AVIVA: a telehealth tool to improve cervical cancer screening in resource-constrained settings
- PMID: 37433694
- PMCID: PMC10347443
- DOI: 10.1136/bmjgh-2023-012311
AVIVA: a telehealth tool to improve cervical cancer screening in resource-constrained settings
Abstract
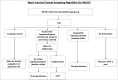
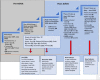
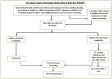
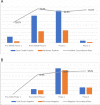
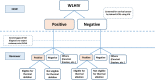
Cervical cancer accounts for 21.7% of all cancer deaths in the sub-Saharan Africa with a case fatality rate of 68%. Nigeria's Federal Ministry of Health has adopted cervical cancer screening (CCS) using visual inspection with acetic acid or Lugol's iodine (VIA/VILI) and cryotherapy treatment for precancerous lesions as the preferred screening and treatment strategy. Using the Exploration, Preparation, Implementation and Sustainment Framework, our study documents our experience during the development, piloting and roll-out of the APIN Public Health Initiatives (APIN)-developed VIA Visual Application (AVIVA) for CCS using the VIA method in 86 APIN-supported health facilities across 7 states in Nigeria. Between December 2019 and June 2022, with the aid of 9 gynaecologists and 133 case finders, a total of 29 262 women living with HIV received VIA-based CCS and 1609 of them were VIA-positive, corresponding to VIA positivity rate of 5.5%. Over the 30 months duration and the 5 phases of CCS scale-up, AVIVA development and expansion, a total of 1247 cases were shared via the AVIVA App (3741 pictures), with 1058 of such cases undergoing expert review, corresponding to a reviewer rate of 84.8%. Overall, the use of the AVIVA App improved both the VIA-positive and VIA-negative concordance rates by 16 percentage points each (26%-42% and 80%-96%, respectively) from baseline to the end of the study. We concluded that the AVIVA App is an innovative tool to improve CCS rates and diagnostic precision by connecting health facility staff and expert reviewers in resource-limited settings.
Keywords: Cancer; HIV; Screening.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
Cervical cancer screening and treatment for PLWHIV: experiences from an innovative program in Nigeria.Reprod Health. 2023 Aug 26;20(1):125. doi: 10.1186/s12978-023-01658-0. Reprod Health. 2023. PMID: 37633884 Free PMC article.
-
A randomized trial comparing the diagnostic accuracy of visual inspection with acetic acid to Visual Inspection with Lugol's Iodine for cervical cancer screening in HIV-infected women.PLoS One. 2015 Apr 7;10(4):e0118568. doi: 10.1371/journal.pone.0118568. eCollection 2015. PLoS One. 2015. PMID: 25849627 Free PMC article. Clinical Trial.
-
Screening for cervical cancer among HIV-positive and HIV-negative women in Cameroon using simultaneous co-testing with careHPV DNA testing and visual inspection enhanced by digital cervicography: Findings of initial screening and one-year follow-up.Gynecol Oncol. 2018 Jan;148(1):118-125. doi: 10.1016/j.ygyno.2017.11.002. Epub 2017 Nov 16. Gynecol Oncol. 2018. PMID: 29153541
-
Accuracy of combinations of visual inspection using acetic acid or lugol iodine to detect cervical precancer: a meta-analysis.BJOG. 2018 Apr;125(5):545-553. doi: 10.1111/1471-0528.14783. Epub 2017 Jul 26. BJOG. 2018. PMID: 28603909
-
Trials and projects on cervical cancer and human papillomavirus prevention in sub-Saharan Africa.Vaccine. 2013 Dec 29;31 Suppl 5:F53-9. doi: 10.1016/j.vaccine.2012.06.070. Vaccine. 2013. PMID: 24331748 Review.
References
-
- World health organization . WHO releases new estimates of the global burden of Cervical cancer associated with HIV. 2020. Available: https://www.who.int/news/item/16-11-2020-who-releases-new-estimates-of-t... [Accessed 5 Aug 2022].
-
- Federal Ministry of Health, Nigeria . Nigeria HIV/AIDS Indicator and Impact Survey (NAIIS). 2018.
-
- Cancer incidence in five continents. volume VIII. IARC Sci Publ 2002:1–781. - PubMed
