Machine learning for prediction of immunotherapeutic outcome in non-small-cell lung cancer based on circulating cytokine signatures
- PMID: 37433717
- PMCID: PMC10347453
- DOI: 10.1136/jitc-2023-006788
Machine learning for prediction of immunotherapeutic outcome in non-small-cell lung cancer based on circulating cytokine signatures
Abstract
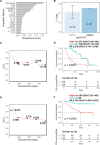
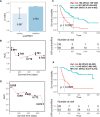
Background: Immune checkpoint inhibitor (ICI) therapy has substantially improved the overall survival (OS) in patients with non-small-cell lung cancer (NSCLC); however, its response rate is still modest. In this study, we developed a machine learning-based platform, namely the Cytokine-based ICI Response Index (CIRI), to predict the ICI response of patients with NSCLC based on the peripheral blood cytokine profiles.
Methods: We enrolled 123 and 99 patients with NSCLC who received anti-PD-1/PD-L1 monotherapy or combined chemotherapy in the training and validation cohorts, respectively. The plasma concentrations of 93 cytokines were examined in the peripheral blood obtained from patients at baseline (pre) and 6 weeks after treatment (early during treatment: edt). Ensemble learning random survival forest classifiers were developed to select feature cytokines and predict the OS of patients undergoing ICI therapy.
Results: Fourteen and 19 cytokines at baseline and on treatment, respectively, were selected to generate CIRI models (namely preCIRI14 and edtCIRI19), both of which successfully identified patients with worse OS in two completely independent cohorts. At the population level, the prediction accuracies of preCIRI14 and edtCIRI19, as indicated by the concordance indices (C-indices), were 0.700 and 0.751 in the validation cohort, respectively. At the individual level, patients with higher CIRI scores demonstrated worse OS [hazard ratio (HR): 0.274 and 0.163, and p<0.0001 and p=0.0044 in preCIRI14 and edtCIRI19, respectively]. By including other circulating and clinical features, improved prediction efficacy was observed in advanced models (preCIRI21 and edtCIRI27). The C-indices in the validation cohort were 0.764 and 0.757, respectively, whereas the HRs of preCIRI21 and edtCIRI27 were 0.141 (p<0.0001) and 0.158 (p=0.038), respectively.
Conclusions: The CIRI model is highly accurate and reproducible in determining the patients with NSCLC who would benefit from anti-PD-1/PD-L1 therapy with prolonged OS and may aid in clinical decision-making before and/or at the early stage of treatment.
Keywords: Biomarkers, Tumor; Biostatistics; Cytokines; Immune Checkpoint Inhibitors; Non-Small Cell Lung Cancer.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: KA received honoraria from AstraZeneka, MSD, Bristol Myers Squibb, Ono and Chugai. YN received honoraria from Ono, Takeda, Eli Lilly, Kyowa Kirin, Boehringer Ingelheim, and AstraZeneca, Bristol Myers Squibb, and research funds from Bristol Myers Squibb. HS received honoraria from Boehringer Ingelheim, Eli Lilly, Pfizer, AstraZeneca, Bristol Myers Squibb, Chugai, and Ono, and research funds from AstraZeneca, Bristol Myers Squibb, Chugai, and Ono. TTagami is an employee of Ajinomoto Co. TKato’s spouse is an employee of Eli Lilly, and he received honoraria from AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Chugai, Daiichi-Sankyo, Eli Lilly, Merck Biopharma, MSD, Novartis, Ono, Pfizer, and Roche, consulting fees from Abbvie, Amgen, AstraZeneca, Beigene, Chugai, Daiichi-Sankyo, Eli Lilly, Glaxo, Merck Biopharma, MSD, Nippon Kayaku, Novartis, Ono, Pfizer, Roche, Taiho, and Takeda, and research funds from Abbvie, Amgen, AstraZeneca, Blueprint, Chugai, Eli Lilly, Haihe, Merck Biopharma, MSD, Novartis, Pfizer, Regeneron, and Takeda. TKondo received honoraria from AstraZeneca, Daiichi-Sankyo, and Otsuka, and research funds from AstraZeneca, Chugai, and Taiho. SM received honoraria from AstraZeneca, Chugai, Boehringer Ingelheim, Taiho, Pfizer, MSD, and Ono. KM received honoraria from AstraZeneka and Chugai. TS received honoraria from Chugai and Bristol Myers Squibb, and research funds from Taiho and BrightPath Biotherapeutics.
Figures




Similar articles
-
Association of the advanced lung cancer inflammation index (ALI) with immune checkpoint inhibitor efficacy in patients with advanced non-small-cell lung cancer.ESMO Open. 2021 Oct;6(5):100254. doi: 10.1016/j.esmoop.2021.100254. Epub 2021 Sep 1. ESMO Open. 2021. PMID: 34481329 Free PMC article.
-
Efficacy of ICI-based treatment in advanced NSCLC patients with PD-L1≥50% who developed EGFR-TKI resistance.Front Immunol. 2023 May 17;14:1161718. doi: 10.3389/fimmu.2023.1161718. eCollection 2023. Front Immunol. 2023. PMID: 37266427 Free PMC article.
-
The effects of antibiotics on the efficacy of immune checkpoint inhibitors in patients with non-small-cell lung cancer differ based on PD-L1 expression.Eur J Cancer. 2021 May;149:73-81. doi: 10.1016/j.ejca.2021.02.040. Epub 2021 Apr 7. Eur J Cancer. 2021. PMID: 33838391
-
Impact of Clinicopathologic Features on the Efficacy of PD-1/PD-L1 Inhibitors in Patients With Previously Treated Non-small-cell Lung Cancer.Clin Lung Cancer. 2018 Mar;19(2):e177-e184. doi: 10.1016/j.cllc.2017.10.018. Epub 2017 Nov 9. Clin Lung Cancer. 2018. PMID: 29175386
-
Comparative efficacy and safety of PD-1/PD-L1 Inhibitors versus platinum-based chemotherapy for the first-line treatment of advanced non-small cell lung cancer: a meta analysis of randomized controlled trials.Pharmacol Res. 2020 Oct;160:105194. doi: 10.1016/j.phrs.2020.105194. Epub 2020 Sep 13. Pharmacol Res. 2020. PMID: 32937178
Cited by
-
Blood-based biomarkers in patients with non-small cell lung cancer treated with immune checkpoint blockade.J Exp Clin Cancer Res. 2024 Mar 16;43(1):82. doi: 10.1186/s13046-024-02969-1. J Exp Clin Cancer Res. 2024. PMID: 38493133 Free PMC article. Review.
-
Construction of a risk prediction model for lung infection after chemotherapy in lung cancer patients based on the machine learning algorithm.Front Oncol. 2024 Aug 9;14:1403392. doi: 10.3389/fonc.2024.1403392. eCollection 2024. Front Oncol. 2024. PMID: 39184040 Free PMC article.
-
Clinical and dynamic circulating cytokines profile features of long-term progression-free survival benefit to immune checkpoint inhibitors in advanced non-small cell lung cancer.Cancer Immunol Immunother. 2025 Apr 17;74(6):173. doi: 10.1007/s00262-025-03984-7. Cancer Immunol Immunother. 2025. PMID: 40244472 Free PMC article.
-
Circulating Biomarkers Predict Immunotherapeutic Response in Hepatocellular Carcinoma Using a Machine Learning Method.J Hepatocell Carcinoma. 2024 Oct 30;11:2133-2144. doi: 10.2147/JHC.S474593. eCollection 2024. J Hepatocell Carcinoma. 2024. PMID: 39493265 Free PMC article.
-
Prediction of Effectiveness and Toxicities of Immune Checkpoint Inhibitors Using Real-World Patient Data.JCO Clin Cancer Inform. 2024 Feb;8:e2300207. doi: 10.1200/CCI.23.00207. JCO Clin Cancer Inform. 2024. PMID: 38427922 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
