A vascular multi-arm multi-stage trial to prevent groin wound surgical site infection: A feasibility survey
- PMID: 37433749
- PMCID: PMC10502270
- DOI: 10.1111/iwj.14170
A vascular multi-arm multi-stage trial to prevent groin wound surgical site infection: A feasibility survey
Abstract
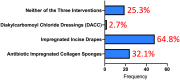
Surgical site infection (SSI) is common following arterial surgery involving a groin incision. There is a lack of evidence regarding interventions to prevent groin wound SSI, therefore, a survey of vascular clinicians was undertaken to assess current opinion and practice, equipoise and feasibility of a randomised controlled trial (RCT). Participants at the Vascular Society of Great Britain and Ireland 2021 Annual Scientific Meeting were surveyed regarding three separate interventions designed to prevent SSI in the groin; impregnated incise drapes, diakylcarbomoyl chloride dressings and antibiotic impregnated collagen sponges. Results were collated via an online survey using the Research Electronic Data Capture platform. Seventy-five participants completed the questionnaire, most were consultant vascular surgeons (50/75, 66.7%). The majority agree that groin wound SSI is a major problem (73/75, 97.3%), and would be content using either of the three interventions (51/61, 83.6%) and had clinical equipoise to randomise patients to any of the three interventions versus standard of care (70/75, 93.3%). There was some reluctance to not use impregnated incise drapes as may be considered "standard of care". Groin wound SSI is perceived as major problem in vascular surgery, and a multicentre RCT of three preventative interventions appears acceptable to vascular surgeons.
Keywords: surgical site infection; vascular surgery.
© 2023 The Authors. International Wound Journal published by Medicalhelplines.com Inc and John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures



References
-
- Centres for Disease Control and prevention . National Healthcare Safety Network (NHSN) Patient Safety Component Manual 2019. https://www.cdc.gov/nhsn/pdfs/pscmanual/pcsmanual_current.pdf. Accessed July 26, 2022
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

