Minimally invasive techniques for lateral maxillary sinus floor elevation: small lateral window and one-stage surgery-a 2-5-year retrospective study
- PMID: 37433766
- PMCID: PMC10336061
- DOI: 10.1038/s41368-023-00233-4
Minimally invasive techniques for lateral maxillary sinus floor elevation: small lateral window and one-stage surgery-a 2-5-year retrospective study
Abstract
This study aimed to introduce a minimally invasive technique for maxillary sinus floor elevation using the lateral approach (lSFE) and to determine the factors that influence the stability of the grafted area in the sinus cavity. Thirty patients (30 implants) treated with lSFE using minimally invasive techniques from 2015 to 2019 were included in the study. Five aspects of the implant (central, mesial, distal, buccal, and palatal bone heights [BHs]) were measured using cone-beam computed tomography (CBCT) before implant surgery, immediately after surgery (T0), 6 months after surgery (T1), and at the last follow-up visit (T2). Patients' characteristics were collected. A small bone window (height, (4.40 ± 0.74) mm; length, (6.26 ± 1.03) mm) was prepared. No implant failed during the follow-up period (3.67 ± 1.75) years. Three of the 30 implants exhibited perforations. Changes in BH of the five aspects of implants showed strong correlations with each other and BH decreased dramatically before second-stage surgery. Residual bone height (RBH) did not significantly influence BH changes, whereas smoking status and type of bone graft materials were the potentially influential factors. During the approximate three-year observation period, lSFE with a minimally invasive technique demonstrated high implant survival rate and limited bone reduction in grafted area. In conclusion, lSFE using minimally invasive techniques was a viable treatment option. Patients who were nonsmokers and whose sinus cavity was filled with deproteinized bovine bone mineral (DBBM) had significantly limited bone resorption in grafted area.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
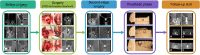


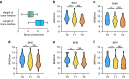
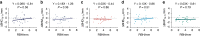
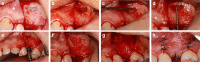
Similar articles
-
Influence of Lateral Windows with Decreased Vertical Height Following Maxillary Sinus Floor Augmentation: A 1-year Clinical and Radiographic Study.Int J Oral Maxillofac Implants. 2018 May/Jun;33(3):661-670. doi: 10.11607/jomi.6213. Int J Oral Maxillofac Implants. 2018. PMID: 29763501
-
Changes in bone graft height and influencing factors after sinus floor augmentation by using the lateral window approach: A clinical retrospective study of 1 to 2 years.J Prosthet Dent. 2023 Sep;130(3):362-368. doi: 10.1016/j.prosdent.2021.10.010. Epub 2021 Nov 29. J Prosthet Dent. 2023. PMID: 34857390
-
Radiographic outcomes of lateral sinus floor elevation with and without bone window repositioning: one-year results of a randomized controlled trial.Int J Oral Maxillofac Surg. 2023 Feb;52(2):255-263. doi: 10.1016/j.ijom.2022.01.021. Epub 2022 Jul 1. Int J Oral Maxillofac Surg. 2023. PMID: 35786525 Clinical Trial.
-
Comparison of Two Different Sizes of Deproteinized Bovine Bone Mineral Particles in Lateral Sinus Floor Elevation With Simultaneous Implant Placement: A Radiographic Study.Clin Oral Implants Res. 2025 Jun;36(6):736-747. doi: 10.1111/clr.14421. Epub 2025 Feb 17. Clin Oral Implants Res. 2025. PMID: 39960188
-
Radiographic outcomes of transcrestal and lateral sinus floor elevation: One-year results of a bi-center, parallel-arm randomized trial.Clin Oral Implants Res. 2019 Sep;30(9):910-919. doi: 10.1111/clr.13497. Epub 2019 Jul 10. Clin Oral Implants Res. 2019. PMID: 31240743 Clinical Trial.
Cited by
-
Biomechanical analysis of maxillary posterior three unit bridge supported misial straight implant and distal tilted implant.Front Bioeng Biotechnol. 2025 Feb 25;13:1546656. doi: 10.3389/fbioe.2025.1546656. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 40070548 Free PMC article.
-
Assessment of guided lateral maxillary sinus lift procedure with simultaneous implant placement using stereolithographic surgical guide: a randomized controlled clinical study.Oral Maxillofac Surg. 2025 May 17;29(1):102. doi: 10.1007/s10006-025-01399-3. Oral Maxillofac Surg. 2025. PMID: 40381068 Free PMC article. Clinical Trial.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

