Effect of down-regulation of let-7c/g on triggering a double-negative feedback loop and promoting restenosis
- PMID: 37433785
- PMCID: PMC10586861
- DOI: 10.1097/CM9.0000000000002763
Effect of down-regulation of let-7c/g on triggering a double-negative feedback loop and promoting restenosis
Abstract
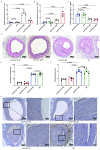
Background: Excessive proliferation and migration of vascular smooth muscle cells (VSMCs) are the main causes of restenosis (RS) in diabetic lower extremity arterial disease (LEAD). However, the relevant pathogenic mechanisms are poorly understood.
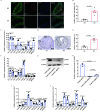
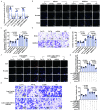
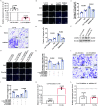
Methods: In this study, we introduced a "two-step injury protocol" rat RS model, which started with the induction of atherosclerosis (AS) and was followed by percutaneous transluminal angioplasty (PTA). Hematoxylin-eosin (HE) staining and immunohistochemistry staining were used to verify the form of RS. Two-step transfection was performed, with the first transfection of Lin28a followed by a second transfection of let-7c and let-7g, to explore the possible mechanism by which Lin28a exerted effects. 5-ethynyl-2΄-deoxyuridine (EdU) and Transwell assay were performed to evaluate the ability of proliferation and migration of VSMCs. Western blotting and quantitative real-time polymerase chain reaction (qRT-PCR) were performed to detect the expression of Lin28a protein and let-7 family members.
Results: Using a combination of in vitro and in vivo experiments, we discovered that let-7c, let-7g, and microRNA98 (miR98) were downstream targets of Lin28a. More importantly, decreased expression of let-7c/let-7g increased Lin28a, leading to further inhibition of let-7c/let-7g. We also found an increased level of let-7d in the RS pathological condition, suggesting that it may function as a protective regulator of the Lin28a/let-7 loop by inhibiting the proliferation and migration of VSMCs.
Conclusion: These findings indicated the presence of a double-negative feedback loop consisting of Lin28a and let-7c/let-7g, which may be responsible for the vicious behavior of VSMCs in RS.
Copyright © 2023 The Chinese Medical Association, produced by Wolters Kluwer, Inc. under the CC-BY-NC-ND license.
Conflict of interest statement
None.
Figures

Similar articles
-
Lin28a up-regulation is associated with the formation of restenosis via promoting proliferation and migration of vascular smooth muscle cells.J Cell Mol Med. 2020 Sep;24(17):9682-9691. doi: 10.1111/jcmm.15506. Epub 2020 Jul 25. J Cell Mol Med. 2020. PMID: 32710472 Free PMC article.
-
C/EBPβ-Lin28a positive feedback loop triggered by C/EBPβ hypomethylation enhances the proliferation and migration of vascular smooth muscle cells in restenosis.Chin Med J (Engl). 2025 Feb 20;138(4):419-429. doi: 10.1097/CM9.0000000000003110. Epub 2024 May 28. Chin Med J (Engl). 2025. PMID: 38809089 Free PMC article.
-
Negative feedback regulation between microRNA let-7g and LOX-1 mediated hypoxia-induced PASMCs proliferation.Biochem Biophys Res Commun. 2017 Jul 8;488(4):655-663. doi: 10.1016/j.bbrc.2017.01.073. Epub 2017 Jan 18. Biochem Biophys Res Commun. 2017. PMID: 28108289
-
Persistent TLR4 Activation Promotes Hepatocellular Carcinoma Growth through Positive Feedback Regulation by LIN28A/Let-7g miRNA.Int J Mol Sci. 2022 Jul 29;23(15):8419. doi: 10.3390/ijms23158419. Int J Mol Sci. 2022. PMID: 35955552 Free PMC article.
-
Aberrant regulation of the LIN28A/LIN28B and let-7 loop in human malignant tumors and its effects on the hallmarks of cancer.Mol Cancer. 2015 Jun 30;14:125. doi: 10.1186/s12943-015-0402-5. Mol Cancer. 2015. PMID: 26123544 Free PMC article. Review.
References
-
- National Clinical Guideline Centre (UK) . Lower limb peripheral arterial disease: Diagnosis and management. London: National Institute for Health and Clinical Excellence: Guidance, 2012.
-
- Low Wang CC Blomster JI Heizer G Berger JS Baumgartner I Fowkes FGR, et al. . Cardiovascular and limb outcomes in patients with diabetes and peripheral artery disease: The EUCLID trial. J Am Coll Cardiol 2018;72: 3274–3284. doi: 10.1016/j.jacc.2018.09.078. - PubMed
-
- Matsuzaki K, Hayashi R, Okabe K, Aramaki-Hattori N, Kishi K. Prognosis of critical limb ischemia: Major vs. minor amputation comparison. Wound Repair Regen 2015;23: 759–764. doi: 10.1111/wrr.12329. - PubMed
-
- Cardaioli P Rigatelli G Dell'avvocata F Giordan M Lisato G Mollo F, et al. . Endovascular treatment of diabetic foot syndrome: Results from a single center prospective registry using mixed coronary and peripheral techniques and equipment. J Interv Cardiol 2011; 24: 562–568. doi: 10.1111/j.1540-8183.2011.00676.x. - PubMed
-
- Tepe G Zeller T Albrecht T Heller S Schwarzwälder U Beregi JP, et al. . Local delivery of paclitaxel to inhibit restenosis during angioplasty of the leg. N Engl J Med 2008;358: 689–699. doi: 10.1056/NEJMoa0706356. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

