Immunogenicity of NVX-CoV2373 heterologous boost against SARS-CoV-2 variants
- PMID: 37433788
- PMCID: PMC10336079
- DOI: 10.1038/s41541-023-00693-z
Immunogenicity of NVX-CoV2373 heterologous boost against SARS-CoV-2 variants
Abstract
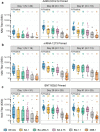
As part of a multicenter study evaluating homologous and heterologous COVID-19 booster vaccines, we assessed the magnitude, breadth, and short-term durability of binding and pseudovirus-neutralizing antibody (PsVNA) responses following a single booster dose of NVX-CoV2373 in adults primed with either Ad26.COV2.S, mRNA-1273, or BNT162b2 vaccines. NVX-CoV2373 as a heterologous booster was immunogenic and associated with no safety concerns through Day 91. Fold-rises in PsVNA titers from baseline (Day 1) to Day 29 were highest for prototypic D614G variant and lowest for more recent Omicron sub-lineages BQ.1.1 and XBB.1. Peak humoral responses against all SARS-CoV-2 variants were lower in those primed with Ad26.COV2.S than with mRNA vaccines. Prior SARS CoV-2 infection was associated with substantially higher baseline PsVNA titers, which remained elevated relative to previously uninfected participants through Day 91. These data support the use of heterologous protein-based booster vaccines as an acceptable alternative to mRNA or adenoviral-based COVID-19 booster vaccines. This trial was conducted under ClinicalTrials.gov: NCT04889209.
© 2023. The Author(s).
Conflict of interest statement
R.L.A., C.P.D.I., C.M.P., D.S., R.P., M.E.D., A.E., H.M.E., M.B., A.C.K., T.M.B., D.D., J.L.A., B.I., S.C., E.R.B., D.J.P., B.C.L., and L.S. declare no competing interests. K.E.L. receives grant awards from Pfizer Inc. (exclusive of the current work). L.A.J.’s institution receives grant funding from NIH, CDC, and Pfizer for vaccine-related assessments, including those of COVID-19 vaccines. R.E.R. serves as an Editor for the journal NPJ Vaccines. A.R.B. has grant funding from Pfizer, Janssen, Merck and Cyanvac for non-COVID-19-related work and serves as a consultant for GSK and Janssen. C.A.R.’s institution has received funds to conduct clinical research from the National Institutes of Health, CDC, BioFire Inc, Genentech, GSK, Janssen, MedImmune, Merck, Micron, Moderna, Novavax, PaxVax, Pfizer, Regeneron, Sanofi-Pasteur. She is co-inventor of patented RSV vaccine technology, which has been licensed to Meissa Vaccines, Inc. J.M.M. has served as a consultant for Merck, Sharp and Dohme for non-Covid-related work. C.J. receives funding from the Bill and Melinda Gates Foundation, N.I.H. and C.D.C., consults for Gilead, Abbvie, Assembly Biosciences and GSK, and receives royalties from UpToDate. M.J.M. has laboratory research and clinical trials contracts for vaccines or MAB vs SARS-CoV-2 with Lilly, Pfizer (exclusive of the current work), and Sanofi; personal fees for Scientific Advisory Board service from Merck, Meissa Vaccines, Inc. and Pfizer. R.N.C. has funding from Cyanvac and HDT Bio to conduct COVID-19-related clinical immunology research. R.C.B. receives funding for vaccine trials from Path Nipah and Pfizer. R.W.F. receives funding to perform clinical trials from Pfizer, Moderna, Astra Zeneca and Emergent Health, and he serves on advisory boards for Johnson & Johnson, Merck, Sanofi Pasteur and Seqirus. S.E. receives funding to her institution from Sanofi Pasteur for a non-COVID-19 vaccine study. K.M.N. holds a grant from Pfizer, without salary support, for a COVID-19 vaccine study, and salary support from the National Institutes of Health (NIH) for work on multiple COVID-19 vaccine trials. D.S.S. is supported by grant awards from NIH/NIAID. P.C.R. and J.H.B. report a pending U.S. Patent Application No. 63/025,918 entitled “Coronavirus RNA vaccines and methods of use”. D.C.M. receives funding from NIH and Moderna for laboratory studies of COVID-19 vaccine antibody responses. We would like to acknowledge Moderna, Inc., Johnson & Johnson/Janssen, and Pfizer/BioNTech Pharmaceuticals for their collaboration, scientific input, and sharing of documents needed to implement this trial. All products were acquired through the government procurement process. All other authors declare no financial or non-financial competing interests.
Figures


References
-
- FDA. Novavax COVID-19 vaccine, adjuvanted. https://www.fda.gov/vaccines-blood-biologics/coronavirus-covid-19-cber-r... (2022).
-
- Munro APS, et al. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): a blinded, multicentre, randomised, controlled, phase 2 trial. Lancet. 2021;398:2258–2276. doi: 10.1016/S0140-6736(21)02717-3. - DOI - PMC - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

