Effects of dialkoxybenzenes against Varroa destructor and identification of 1-allyloxy-4-propoxybenzene as a promising acaricide candidate
- PMID: 37433810
- PMCID: PMC10336134
- DOI: 10.1038/s41598-023-38187-6
Effects of dialkoxybenzenes against Varroa destructor and identification of 1-allyloxy-4-propoxybenzene as a promising acaricide candidate
Abstract
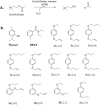
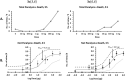
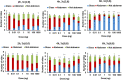
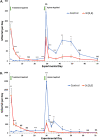
The honey bee is responsible for pollination of a large proportion of crop plants, but the health of honey bee populations has been challenged by the parasitic mite Varroa destructor. Mite infestation is the main cause of colony losses during the winter months, which causes significant economic challenges in apiculture. Treatments have been developed to control the spread of varroa. However, many of these treatments are no longer effective due to acaricide resistance. In a search of varroa-active compounds, we tested the effect of dialkoxybenzenes on the mite. A structure-activity relationship revealed that 1-allyloxy-4-propoxybenzene is most active of a series of dialkoxybenzenes tested. We found that three compounds (1-allyloxy-4-propoxybenzene, 1,4-diallyloxybenzene and 1,4-dipropoxybenzene) cause paralysis and death of adult varroa mites, whereas the previously discovered compound, 1,3-diethoxybenzene, which alters host choice of adult mites in certain conditions, did not cause paralysis. Since paralysis can be caused by inhibition of acetylcholinesterase (AChE), a ubiquitous enzyme in the nervous system of animals, we tested dialkoxybenzenes on human, honey bee and varroa AChE. These tests revealed that 1-allyloxy-4-propoxybenzene had no effects on AChE, which leads us to conclude that 1-allyloxy-4-propoxybenzene does not exert its paralytic effect on mites through AChE. In addition to paralysis, the most active compounds affected the ability of the mites to find and remain at the abdomen of host bees provided during assays. A test of 1-allyloxy-4-propoxybenzene in the field, during the autumn of 2019 in two locations, showed that this compound has promise in the treatment of varroa infestations.
© 2023. The Author(s).
Conflict of interest statement
EP has signed the technology over to SFU. Patent application by (applicants) SFU and ARO; inventors are EP and V. Soroker, application filed (PCT/CA2019/050908). Only experiments performed in 2018 were included in the patent. The remaining authors do not have any competing interests.
Figures




Similar articles
-
The efficacy of 1-allyloxy-4-propoxybenzene (3c{3,6}) against Varroa destructor mites in honey bee colonies from Maryland, USA.J Insect Sci. 2024 May 1;24(3):9. doi: 10.1093/jisesa/iead111. J Insect Sci. 2024. PMID: 38805649 Free PMC article.
-
Varroa destructor: A Complex Parasite, Crippling Honey Bees Worldwide.Trends Parasitol. 2020 Jul;36(7):592-606. doi: 10.1016/j.pt.2020.04.004. Epub 2020 May 23. Trends Parasitol. 2020. PMID: 32456963 Review.
-
Voltage-gated chloride channel blocker DIDS as an acaricide for Varroa mites.Pestic Biochem Physiol. 2020 Jul;167:104603. doi: 10.1016/j.pestbp.2020.104603. Epub 2020 May 13. Pestic Biochem Physiol. 2020. PMID: 32527437
-
Acaricide treatment affects viral dynamics in Varroa destructor-infested honey bee colonies via both host physiology and mite control.Appl Environ Microbiol. 2012 Jan;78(1):227-35. doi: 10.1128/AEM.06094-11. Epub 2011 Oct 21. Appl Environ Microbiol. 2012. PMID: 22020517 Free PMC article.
-
Reproductive biology of Varroa destructor in Africanized honey bees (Apis mellifera).Exp Appl Acarol. 2010 Apr;50(4):281-97. doi: 10.1007/s10493-009-9325-4. Epub 2009 Oct 23. Exp Appl Acarol. 2010. PMID: 19851876 Review.
Cited by
-
The efficacy of 1-allyloxy-4-propoxybenzene (3c{3,6}) against Varroa destructor mites in honey bee colonies from Maryland, USA.J Insect Sci. 2024 May 1;24(3):9. doi: 10.1093/jisesa/iead111. J Insect Sci. 2024. PMID: 38805649 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

