On the mechanisms of lysis triggered by perturbations of bacterial cell wall biosynthesis
- PMID: 37433811
- PMCID: PMC10336097
- DOI: 10.1038/s41467-023-39723-8
On the mechanisms of lysis triggered by perturbations of bacterial cell wall biosynthesis
Abstract
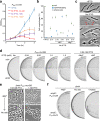
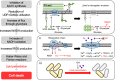
Inhibition of bacterial cell wall synthesis by antibiotics such as β-lactams is thought to cause explosive lysis through loss of cell wall integrity. However, recent studies on a wide range of bacteria have suggested that these antibiotics also perturb central carbon metabolism, contributing to death via oxidative damage. Here, we genetically dissect this connection in Bacillus subtilis perturbed for cell wall synthesis, and identify key enzymatic steps in upstream and downstream pathways that stimulate the generation of reactive oxygen species through cellular respiration. Our results also reveal the critical role of iron homeostasis for the oxidative damage-mediated lethal effects. We show that protection of cells from oxygen radicals via a recently discovered siderophore-like compound uncouples changes in cell morphology normally associated with cell death, from lysis as usually judged by a phase pale microscopic appearance. Phase paling appears to be closely associated with lipid peroxidation.
© 2023. Crown.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

