YF17D-vectored Ebola vaccine candidate protects mice against lethal surrogate Ebola and yellow fever virus challenge
- PMID: 37433816
- PMCID: PMC10336040
- DOI: 10.1038/s41541-023-00699-7
YF17D-vectored Ebola vaccine candidate protects mice against lethal surrogate Ebola and yellow fever virus challenge
Abstract
Ebola virus (EBOV) and related filoviruses such as Sudan virus (SUDV) threaten global public health. Effective filovirus vaccines are available only for EBOV, yet restricted to emergency use considering a high reactogenicity and demanding logistics. Here we present YF-EBO, a live YF17D-vectored dual-target vaccine candidate expressing EBOV glycoprotein (GP) as protective antigen. Safety of YF-EBO in mice was further improved over that of parental YF17D vaccine. A single dose of YF-EBO was sufficient to induce high levels of EBOV GP-specific antibodies and cellular immune responses, that protected against lethal infection using EBOV GP-pseudotyped recombinant vesicular stomatitis virus (rVSV-EBOV) in interferon-deficient (Ifnar-/-) mice as surrogate challenge model. Concomitantly induced yellow fever virus (YFV)-specific immunity protected Ifnar-/- mice against intracranial YFV challenge. YF-EBO could thus help to simultaneously combat both EBOV and YFV epidemics. Finally, we demonstrate how to target other highly pathogenic filoviruses such as SUDV at the root of the 2022 outbreak in Uganda.
© 2023. The Author(s).
Conflict of interest statement
K.D., L.S.-F., V.L., and J.N. are mentioned as inventors on patent applications related to the discovery and use of YF17D-vectored filovirus vaccines. The other authors declare no competing interests.
Figures

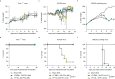


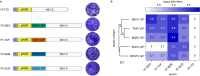
References
-
- Henao-Restrepo AM, et al. Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ça Suffit!) Lancet. 2017;389:505–518. doi: 10.1016/S0140-6736(16)32621-6. - DOI - PMC - PubMed
-
- WHO. Preliminary results on the efficacy of rVSV-ZEBOV-GP Ebola vaccine using the ring vaccination strategy in the control of an Ebola outbreak in the Democratic Republic of the Congo: an example of integration of research into epidemic response. https://www.who.int/publications/m/item/preliminary-results-on-the-effic... (2019).
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

