Architectural basis for cylindrical self-assembly governing Plk4-mediated centriole duplication in human cells
- PMID: 37433832
- PMCID: PMC10336005
- DOI: 10.1038/s42003-023-05067-8
Architectural basis for cylindrical self-assembly governing Plk4-mediated centriole duplication in human cells
Erratum in
-
Author Correction: Architectural basis for cylindrical self-assembly governing Plk4-mediated centriole duplication in human cells.Commun Biol. 2023 Jul 27;6(1):784. doi: 10.1038/s42003-023-05162-w. Commun Biol. 2023. PMID: 37500946 Free PMC article. No abstract available.
Abstract
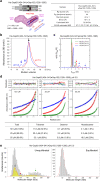
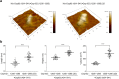
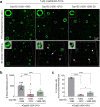
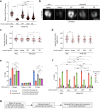
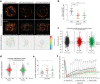
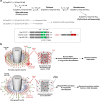
Proper organization of intracellular assemblies is fundamental for efficient promotion of biochemical processes and optimal assembly functionality. Although advances in imaging technologies have shed light on how the centrosome is organized, how its constituent proteins are coherently architected to elicit downstream events remains poorly understood. Using multidisciplinary approaches, we showed that two long coiled-coil proteins, Cep63 and Cep152, form a heterotetrameric building block that undergoes a stepwise formation into higher molecular weight complexes, ultimately generating a cylindrical architecture around a centriole. Mutants defective in Cep63•Cep152 heterotetramer formation displayed crippled pericentriolar Cep152 organization, polo-like kinase 4 (Plk4) relocalization to the procentriole assembly site, and Plk4-mediated centriole duplication. Given that the organization of pericentriolar materials (PCM) is evolutionarily conserved, this work could serve as a model for investigating the structure and function of PCM in other species, while offering a new direction in probing the organizational defects of PCM-related human diseases.
© 2023. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Conduit PT, Wainman A, Raff JW. Centrosome function and assembly in animal cells. Nat. Rev. Mol. Cell Biol. 2015;16:611–624. - PubMed
-
- Lawo S, Hasegan M, Gupta GD, Pelletier L. Subdiffraction imaging of centrosomes reveals higher-order organizational features of pericentriolar material. Nat. Cell Biol. 2012;14:1148–1158. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

