Oxidative stress increases in liver of lactating rats after BPF-low-dose exposure: perinatal effects in the offspring
- PMID: 37433837
- PMCID: PMC10336036
- DOI: 10.1038/s41598-023-38434-w
Oxidative stress increases in liver of lactating rats after BPF-low-dose exposure: perinatal effects in the offspring
Abstract
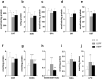
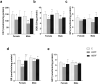
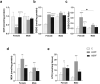
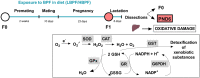
Bisphenol F (BPF) is replacing Bisphenol A (BPA) in the manufacture of products due to endocrine-disrupting effects. BPF monomers can also be released into the environment and enter the food chain, resulting in human exposure to low doses. Since bisphenols are primarily metabolized by the liver, this organ is more vulnerable to lower doses of bisphenols than others. Exposure during prenatal development may increase the risk of diseases in adulthood. The aim was to evaluate whether BPF administration could generate oxidative stress in liver of lactating rats, and whether these effects may be also observed in female and male postnatal day 6 (PND6) offspring. Long Evans rats received oral treatment: Control, BPF-low-dose (LBPF) 0.0365 mg/kg b.w./day, and BPF-high-dose (HBPF) 3.65 mg/kg b.w./day. The levels of antioxidant enzymes (CAT, SOD, GR, GPx and GST), glutathione system (GSH, GSSG) and lipid damage markers (MDA, LPO) were measured using colorimetric methods in liver of both lactating dams and in PND6 offspring. Mean values were analyzed using Prism-7. LBPF affected liver defense mechanisms (antioxidant enzymes and glutathione system), increasing ROS levels and producing lipid peroxidation in lactating dams. Similar effects were found in female and male PND6 offspring as a consequence of perinatal exposure.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
DINCH Exposure Triggers Inflammatory, Oxidative, and Apoptotic Pathways in the Liver of Long-Evans Lactating Rats and Their Offspring.Int J Mol Sci. 2024 Dec 3;25(23):13017. doi: 10.3390/ijms252313017. Int J Mol Sci. 2024. PMID: 39684727 Free PMC article.
-
Activation of NLRP3 Inflammasome in Liver of Long Evans Lactating Rats and Its Perinatal Effects in the Offspring after Bisphenol F Exposure.Int J Mol Sci. 2023 Sep 15;24(18):14129. doi: 10.3390/ijms241814129. Int J Mol Sci. 2023. PMID: 37762434 Free PMC article.
-
Low Dose of BPA Induces Liver Injury through Oxidative Stress, Inflammation and Apoptosis in Long-Evans Lactating Rats and Its Perinatal Effect on Female PND6 Offspring.Int J Mol Sci. 2023 Feb 26;24(5):4585. doi: 10.3390/ijms24054585. Int J Mol Sci. 2023. PMID: 36902016 Free PMC article.
-
Effects of perinatal exposure to BPA, BPF and BPAF on liver function in male mouse offspring involving in oxidative damage and metabolic disorder.Environ Pollut. 2019 Apr;247:935-943. doi: 10.1016/j.envpol.2019.01.116. Epub 2019 Jan 30. Environ Pollut. 2019. PMID: 30823348
-
Prenatal exposure to bisphenols affects pregnancy outcomes and offspring development in rats.Chemosphere. 2021 Aug;276:130118. doi: 10.1016/j.chemosphere.2021.130118. Epub 2021 Feb 26. Chemosphere. 2021. PMID: 33714148
Cited by
-
A novel bellidifolin intervention mitigates nonalcoholic fatty liver disease-like changes induced by bisphenol F.J Biomed Res. 2024 Feb 23;38(5):451-463. doi: 10.7555/JBR.37.20230169. J Biomed Res. 2024. PMID: 38808572 Free PMC article.
-
DINCH Exposure Triggers Inflammatory, Oxidative, and Apoptotic Pathways in the Liver of Long-Evans Lactating Rats and Their Offspring.Int J Mol Sci. 2024 Dec 3;25(23):13017. doi: 10.3390/ijms252313017. Int J Mol Sci. 2024. PMID: 39684727 Free PMC article.
-
Effects of Prenatal Exposure to Bisphenol A Substitutes, Bisphenol S and Bisphenol F, on Offspring's Health: Evidence from Epidemiological and Experimental Studies.Biomolecules. 2023 Nov 5;13(11):1616. doi: 10.3390/biom13111616. Biomolecules. 2023. PMID: 38002298 Free PMC article. Review.
-
Activation of NLRP3 Inflammasome in Liver of Long Evans Lactating Rats and Its Perinatal Effects in the Offspring after Bisphenol F Exposure.Int J Mol Sci. 2023 Sep 15;24(18):14129. doi: 10.3390/ijms241814129. Int J Mol Sci. 2023. PMID: 37762434 Free PMC article.
-
The effects of ketoprofen and meloxicam on oxidative stress through the glutathione pathway after ketamine-xylazine anesthesia and ulcer induction in rats: A comparative study.Vet Anim Sci. 2024 Jul 14;25:100377. doi: 10.1016/j.vas.2024.100377. eCollection 2024 Sep. Vet Anim Sci. 2024. PMID: 39130674 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

