Pancreatic ductal adenocarcinoma induces neural injury that promotes a transcriptomic and functional repair signature by peripheral neuroglia
- PMID: 37433986
- PMCID: PMC10880465
- DOI: 10.1038/s41388-023-02775-7
Pancreatic ductal adenocarcinoma induces neural injury that promotes a transcriptomic and functional repair signature by peripheral neuroglia
Abstract
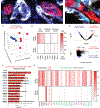
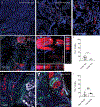
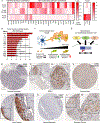
Perineural invasion (PNI) is the phenomenon whereby cancer cells invade the space surrounding nerves. PNI occurs frequently in epithelial malignancies, but is especially characteristic of pancreatic ductal adenocarcinoma (PDAC). The presence of PNI portends an increased incidence of local recurrence, metastasis and poorer overall survival. While interactions between tumor cells and nerves have been investigated, the etiology and initiating cues for PNI development is not well understood. Here, we used digital spatial profiling to reveal changes in the transcriptome and to allow for a functional analysis of neural-supportive cell types present within the tumor-nerve microenvironment of PDAC during PNI. We found that hypertrophic tumor-associated nerves within PDAC express transcriptomic signals of nerve damage including programmed cell death, Schwann cell proliferation signaling pathways, as well as macrophage clearance of apoptotic cell debris by phagocytosis. Moreover, we identified that neural hypertrophic regions have increased local neuroglial cell proliferation which was tracked using EdU tumor labeling in KPC mice, as well as frequent TUNEL positivity, suggestive of a high turnover rate. Functional calcium imaging studies using human PDAC organotypic slices confirmed nerve bundles had neuronal activity, as well as contained NGFR+ cells with high sustained calcium levels, which are indicative of apoptosis. This study reveals a common gene expression pattern that characterizes solid tumor-induced damage to local nerves. These data provide new insights into the pathobiology of the tumor-nerve microenvironment during PDAC as well as other gastrointestinal cancers.
© 2023. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
COMPETING INTERESTS
The authors declare no competing financial interests.
Competing Interests statement: The authors have nothing to disclose.
Figures


Update of
-
Pancreatic Ductal Adenocarcinoma Induces Neural Injury that Promotes a Transcriptomic and Functional Repair Signature by Peripheral Neuroglia.Res Sq [Preprint]. 2023 Mar 28:rs.3.rs-2715023. doi: 10.21203/rs.3.rs-2715023/v1. Res Sq. 2023. Update in: Oncogene. 2023 Aug;42(34):2536-2546. doi: 10.1038/s41388-023-02775-7. PMID: 37034696 Free PMC article. Updated. Preprint.
Similar articles
-
Pancreatic Ductal Adenocarcinoma Induces Neural Injury that Promotes a Transcriptomic and Functional Repair Signature by Peripheral Neuroglia.Res Sq [Preprint]. 2023 Mar 28:rs.3.rs-2715023. doi: 10.21203/rs.3.rs-2715023/v1. Res Sq. 2023. Update in: Oncogene. 2023 Aug;42(34):2536-2546. doi: 10.1038/s41388-023-02775-7. PMID: 37034696 Free PMC article. Updated. Preprint.
-
Tumor-neuroglia interaction promotes pancreatic cancer metastasis.Theranostics. 2020 Apr 6;10(11):5029-5047. doi: 10.7150/thno.42440. eCollection 2020. Theranostics. 2020. PMID: 32308766 Free PMC article.
-
Tenascin C in the Tumor-Nerve Microenvironment Enhances Perineural Invasion and Correlates With Locoregional Recurrence in Pancreatic Ductal Adenocarcinoma.Pancreas. 2020 Mar;49(3):442-454. doi: 10.1097/MPA.0000000000001506. Pancreas. 2020. PMID: 32132519
-
Targeting the Cancer-Neuronal Crosstalk in the Pancreatic Cancer Microenvironment.Int J Mol Sci. 2023 Oct 8;24(19):14989. doi: 10.3390/ijms241914989. Int J Mol Sci. 2023. PMID: 37834436 Free PMC article. Review.
-
Cellular and molecular mechanisms of perineural invasion of pancreatic ductal adenocarcinoma.Cancer Commun (Lond). 2021 Aug;41(8):642-660. doi: 10.1002/cac2.12188. Epub 2021 Jul 15. Cancer Commun (Lond). 2021. PMID: 34264020 Free PMC article. Review.
Cited by
-
Experimental models of pancreas cancer: what has been the impact for precision medicine?J Clin Invest. 2025 Aug 15;135(16):e191945. doi: 10.1172/JCI191945. eCollection 2025 Aug 15. J Clin Invest. 2025. PMID: 40829173 Free PMC article. Review.
-
Advances in spatial transcriptomics and its applications in cancer research.Mol Cancer. 2024 Jun 20;23(1):129. doi: 10.1186/s12943-024-02040-9. Mol Cancer. 2024. PMID: 38902727 Free PMC article. Review.
-
Spatial Transcriptomics Reveals Novel Mechanisms Involved in Perineural Invasion in Pancreatic Ductal Adenocarcinomas.Cancers (Basel). 2025 Mar 1;17(5):852. doi: 10.3390/cancers17050852. Cancers (Basel). 2025. PMID: 40075699 Free PMC article.
-
Progress of single-cell RNA sequencing combined with spatial transcriptomics in tumour microenvironment and treatment of pancreatic cancer.J Transl Med. 2024 Jun 12;22(1):563. doi: 10.1186/s12967-024-05307-3. J Transl Med. 2024. PMID: 38867230 Free PMC article. Review.
-
The supporting role of Schwann cells in perineural invasion of pancreatic ductal adenocarcinoma.Front Pharmacol. 2025 Jun 11;16:1540027. doi: 10.3389/fphar.2025.1540027. eCollection 2025. Front Pharmacol. 2025. PMID: 40567365 Free PMC article. Review.

