Screening of placenta accreta spectrum disorder using maternal serum biomarkers and clinical indicators: a case-control study
- PMID: 37434137
- PMCID: PMC10334543
- DOI: 10.1186/s12884-023-05784-2
Screening of placenta accreta spectrum disorder using maternal serum biomarkers and clinical indicators: a case-control study
Abstract
Background: Placenta accreta spectrum (PAS) disorder is a major cause of postpartum hemorrhage-associated maternal and fetal death, and novel methods for PAS screening are urgently needed for clinical application.
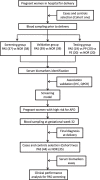
Methods: The purpose of this study was to develop new methods for PAS screening using serum biomarkers and clinical indicators. A total of 95 PAS cases and 137 controls were enrolled in a case-control study as cohort one, and 44 PAS cases and 35 controls in a prospective nested case-control study were enrolled as cohort two. All subjects were pregnant women of Chinese Han population. Biomarkers for PAS from maternal blood samples were screened based on high-throughput immunoassay and were further validated in three phases of cohort one. Screening models for PAS were generated using maternal serum biomarkers and clinical indicators, and were validated in two cohorts. The expression levels of biomarkers were analyzed using histopathological and immunohistochemical (IHC) techniques, and gene expression was examined by QPCR in the human placenta. Binary logistic regression models were built, and the area under the curve (AUC), sensitivity, specificity, and Youden index were calculated. Statistical analyses and model building were performed in SPSS and graphs were generated in GraphPad Prism. The independent-sample t test was used to compare numerical data between two groups. For nonparametric variables, a Mann-Whitney U test or a X2 test was used.
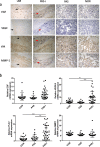
Results: The results demonstrated that the serum levels of matrix metalloproteinase-1 (MMP-1), epidermal growth factor (EGF), and vascular endothelial growth factor-A (VEGF-A) were consistently higher, while the level of tissue-type plasminogen activator (tPA) was significantly lower in PAS patients compared with normal term controls and patients with pre-eclampsia (PE) and placenta previa (PP). IHC and QPCR analysis confirmed that the expression of the identified biomarkers significantly changed during the third trimester in human placenta. The generated screening model combining serum biomarkers and clinical indicators detected 87% of PAS cases with AUC of 0.94.
Conclusions: Serum biomarkers can be used for PAS screening with low expense and high clinical performance; therefore, it may help to develop a practicable method for clinical prenatal PAS screening.
Keywords: Biomarkers; Placenta accreta spectrum; Screening model.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Antenatal maternal serum biomarkers as a predictor for placenta accreta spectrum disorders.Placenta. 2024 Dec;158:62-68. doi: 10.1016/j.placenta.2024.10.002. Epub 2024 Oct 3. Placenta. 2024. PMID: 39369621
-
Predictive value of vascular endothelial growth factor and placenta growth factor for placenta accreta spectrum.J Obstet Gynaecol. 2022 Jul;42(5):900-905. doi: 10.1080/01443615.2021.1955337. Epub 2021 Sep 24. J Obstet Gynaecol. 2022. PMID: 34558384
-
Evaluation of maternal serum VEGF, TNF-alpha, IL-4, and IL-10 levels in differentiating placenta accreta spectrum from isolated placenta previa.Cytokine. 2024 Apr;176:156513. doi: 10.1016/j.cyto.2024.156513. Epub 2024 Jan 22. Cytokine. 2024. PMID: 38262117
-
Evaluation of maternal serum protein biomarkers in the prenatal evaluation of placenta accreta spectrum: A systematic scoping review.Acta Obstet Gynecol Scand. 2024 Dec;103(12):2335-2347. doi: 10.1111/aogs.14918. Epub 2024 Jul 14. Acta Obstet Gynecol Scand. 2024. PMID: 39004916 Free PMC article.
-
Association between placenta accreta spectrum and third-trimester serum levels of vascular endothelial growth factor, placental growth factor, and soluble Fms-like tyrosine kinase-1: A meta-analysis.J Obstet Gynaecol Res. 2022 Sep;48(9):2363-2376. doi: 10.1111/jog.15330. Epub 2022 Jun 20. J Obstet Gynaecol Res. 2022. PMID: 35726123
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

