Pathophysiology and probable etiology of cerebral small vessel disease in vascular dementia and Alzheimer's disease
- PMID: 37434208
- PMCID: PMC10334598
- DOI: 10.1186/s13024-023-00640-5
Pathophysiology and probable etiology of cerebral small vessel disease in vascular dementia and Alzheimer's disease
Abstract
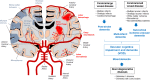
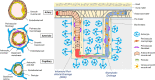
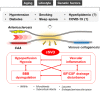
Vascular cognitive impairment and dementia (VCID) is commonly caused by vascular injuries in cerebral large and small vessels and is a key driver of age-related cognitive decline. Severe VCID includes post-stroke dementia, subcortical ischemic vascular dementia, multi-infarct dementia, and mixed dementia. While VCID is acknowledged as the second most common form of dementia after Alzheimer's disease (AD) accounting for 20% of dementia cases, VCID and AD frequently coexist. In VCID, cerebral small vessel disease (cSVD) often affects arterioles, capillaries, and venules, where arteriolosclerosis and cerebral amyloid angiopathy (CAA) are major pathologies. White matter hyperintensities, recent small subcortical infarcts, lacunes of presumed vascular origin, enlarged perivascular space, microbleeds, and brain atrophy are neuroimaging hallmarks of cSVD. The current primary approach to cSVD treatment is to control vascular risk factors such as hypertension, dyslipidemia, diabetes, and smoking. However, causal therapeutic strategies have not been established partly due to the heterogeneous pathogenesis of cSVD. In this review, we summarize the pathophysiology of cSVD and discuss the probable etiological pathways by focusing on hypoperfusion/hypoxia, blood-brain barriers (BBB) dysregulation, brain fluid drainage disturbances, and vascular inflammation to define potential diagnostic and therapeutic targets for cSVD.
Keywords: Arteriolosclerosis; Blood–brain barriers (BBB); Cerebral amyloid angiopathy (CAA); Cerebral small vessel disease (cSVD); Glymphatic drainage; Hypoperfusion/Hypoxia; Intramural periarterial drainage (IPAD); Vascular cognitive impairment and dementia (VCID); Vascular inflammation.
© 2023. The Author(s).
Conflict of interest statement
G.B. is an employee of SciNeuro Pharmaceuticals. Other authors declare no competing interests.
Figures
References
-
- O’Brien JT, Erkinjuntti T, Reisberg B, Roman G, Sawada T, Pantoni L, Bowler JV, Ballard C, DeCarli C, Gorelick PB, et al. Vascular cognitive impairment. Lancet Neurol. 2003;2:89–98. - PubMed
-
- Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42:2672–2713. - PMC - PubMed
-
- Skrobot OA, O’Brien J, Black S, Chen C, DeCarli C, Erkinjuntti T, Ford GA, Kalaria RN, Pantoni L, Pasquier F, et al. The Vascular Impairment of Cognition Classification Consensus Study. Alzheimers Dement. 2017;13:624–33. - PubMed
-
- Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. Lancet Neurol. 2009;8:1006–1018. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

