NLRP3-GABA signaling pathway contributes to the pathogenesis of impulsive-like behaviors and cognitive deficits in aged mice
- PMID: 37434240
- PMCID: PMC10337164
- DOI: 10.1186/s12974-023-02845-3
NLRP3-GABA signaling pathway contributes to the pathogenesis of impulsive-like behaviors and cognitive deficits in aged mice
Abstract
Background: Perioperative neurocognitive disorders (PND), such as delirium and cognitive impairment, are commonly encountered complications in aged patients. The inhibitory neurotransmitter γ-aminobutyric acid (GABA) is aberrantly synthesized from reactive astrocytes following inflammatory stimulation and is implicated in the pathophysiology of neurodegenerative diseases. Additionally, the activation of NOD-like receptor protein 3 (NLRP3) inflammasome is involved in PND. Herein, we aimed to investigate whether the NLRP3-GABA signaling pathway contributes to the pathogenesis of aging mice's PND.
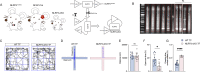
Methods: 24-month-old C57BL/6 and astrocyte-specific NLRP3 knockout male mice were used to establish a PND model via tibial fracture surgery. The monoamine oxidase-B (MAOB) inhibitor selegiline (1 mg/kg) was intraperitoneally administered once a day for 7 days after the surgery. PND, including impulsive-like behaviors and cognitive impairment, was evaluated by open field test, elevated plus maze, and fear conditioning. Thereafter, pathological changes of neurodegeneration were explored by western blot and immunofluorescence assays.
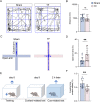
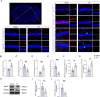
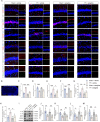
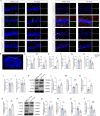
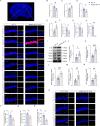
Results: Selegiline administration significantly ameliorated TF-induced impulsive-like behaviors and reduced excessive GABA production in reactive hippocampal astrocytes. Moreover, astrocyte-specific NLRP3 knockout mice reversed TF-induced impulsive-like and cognitive impairment behaviors, decreased GABA levels in reactive astrocytes, ameliorated NLRP3-associated inflammatory responses during the early stage, and restored neuronal degeneration in the hippocampus.
Conclusions: Our findings suggest that anesthesia and surgical procedures trigger neuroinflammation and cognitive deficits, which may be due to NLRP3-GABA activation in the hippocampus of aged mice.
Keywords: Astrocyte; GABA; NLRP3; Perioperative neurocognitive disorders; Selegiline.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Inhibition of the P38 MAPK/NLRP3 pathway mitigates cognitive dysfunction and mood alterations in aged mice after abdominal surgery plus sevoflurane.Brain Res Bull. 2024 Oct 15;217:111059. doi: 10.1016/j.brainresbull.2024.111059. Epub 2024 Aug 30. Brain Res Bull. 2024. PMID: 39216556
-
Activation of the hippocampal CA1 astrocyte Gq and Gi G protein-coupled receptors exerts a protective effect against attention deficit hyperactivity disorder.Int Immunopharmacol. 2025 Apr 16;152:114382. doi: 10.1016/j.intimp.2025.114382. Epub 2025 Mar 5. Int Immunopharmacol. 2025. PMID: 40049085
-
The PGC-1α/ERRα/ULK1 pathway contributes to Perioperative neurocognitive disorders by inducing mitochondrial dysfunction and activating NLRP3 inflammasome in aged mice.Neuropharmacology. 2024 Dec 1;260:110119. doi: 10.1016/j.neuropharm.2024.110119. Epub 2024 Aug 27. Neuropharmacology. 2024. PMID: 39197819
-
α5GABAA receptor: A potential therapeutic target for perioperative neurocognitive disorders, a review of preclinical studies.Brain Res Bull. 2023 Dec;205:110821. doi: 10.1016/j.brainresbull.2023.110821. Epub 2023 Nov 18. Brain Res Bull. 2023. PMID: 37984621 Review.
-
Recent Advances in the Mechanisms of Postoperative Neurocognitive Dysfunction: A Narrative Review.Biomedicines. 2025 Jan 7;13(1):115. doi: 10.3390/biomedicines13010115. Biomedicines. 2025. PMID: 39857699 Free PMC article. Review.
Cited by
-
Expression of microRNA induced by postoperative delirium-like behavior is associated with long-term default mode network disruption: Sequencing and a secondary analysis of resting-state fMRI data.CNS Neurosci Ther. 2024 Sep;30(9):e70038. doi: 10.1111/cns.70038. CNS Neurosci Ther. 2024. PMID: 39317458 Free PMC article.
-
Updated insights into the NLRP3 inflammasome in postoperative cognitive dysfunction: emerging mechanisms and treatments.Front Aging Neurosci. 2024 Sep 30;16:1480502. doi: 10.3389/fnagi.2024.1480502. eCollection 2024. Front Aging Neurosci. 2024. PMID: 39411285 Free PMC article. Review.
-
Role of glia in delirium: proposed mechanisms and translational implications.Mol Psychiatry. 2025 Mar;30(3):1138-1147. doi: 10.1038/s41380-024-02801-4. Epub 2024 Oct 27. Mol Psychiatry. 2025. PMID: 39463449 Free PMC article. Review.
-
Abscisic Acid Rescues Behavior in Adult Female Mice in Attention Deficit Disorder with Hyperactivity Model of Dopamine Depletion by Regulating Microglia and Increasing Vesicular GABA Transporter Expression.J Neuroimmune Pharmacol. 2025 Apr 16;20(1):39. doi: 10.1007/s11481-025-10186-6. J Neuroimmune Pharmacol. 2025. PMID: 40234284 Free PMC article.
-
Peripheral inflammation and neurocognitive impairment: correlations, underlying mechanisms, and therapeutic implications.Front Aging Neurosci. 2023 Nov 29;15:1305790. doi: 10.3389/fnagi.2023.1305790. eCollection 2023. Front Aging Neurosci. 2023. PMID: 38094503 Free PMC article. Review.
References
-
- Yu J, Rawtaer I, Feng L, Kua EH, Mahendran R. The functional and structural connectomes associated with geriatric depression and anxiety symptoms in mild cognitive impairment: cross-syndrome overlap and generalization. Prog Neuropsychopharmacol Biol Psychiatry. 2021;110:110329. doi: 10.1016/j.pnpbp.2021.110329. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

