Efficacy and safety of FDA-approved IDH inhibitors in the treatment of IDH mutated acute myeloid leukemia: a systematic review and meta-analysis
- PMID: 37434249
- PMCID: PMC10334617
- DOI: 10.1186/s13148-023-01529-2
Efficacy and safety of FDA-approved IDH inhibitors in the treatment of IDH mutated acute myeloid leukemia: a systematic review and meta-analysis
Abstract
Objective: To systematically evaluate the efficacy and safety of FDA-approved isocitrate dehydrogenase (IDH) inhibitors in the treatment of IDH-mutated acute myeloid leukemia (AML).
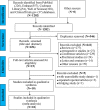
Methods: We used R software to conduct a meta-analysis of prospective clinical trials of IDH inhibitors in the treatment of IDH-mutated AML published in PubMed, Embase, Clinical Trials, Cochrane Library and Web of Science from inception to November 15th, 2022.
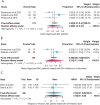
Results: A total of 1109 IDH-mutated AML patients from 10 articles (11 cohorts) were included in our meta-analysis. The CR rate, ORR rate, 2-year survival (OS) rate and 2-year event-free survival (EFS) rate of newly diagnosed IDH-mutated AML (715 patients) were 47%, 65%, 45% and 29%, respectively. The CR rate, ORR rate, 2-year OS rate, median OS and median EFS of relapsed or refractory (R/R) IDH-mutated AML (394 patients) were 21%, 40%, 15%, 8.21 months and 4.73 months, respectively. Gastrointestinal adverse events were the most frequently occurring all-grade adverse events and hematologic adverse events were the most frequently occurring ≥ grade 3 adverse events.
Conclusion: IDH inhibitor is a promising treatment for R/R AML patients with IDH mutations. For patients with newly diagnosed IDH-mutated AML, IDH inhibitors may not be optimal therapeutic agents due to low CR rates. The safety of IDH inhibitors is controllable, but physicians should always pay attention to and manage the differentiation syndrome adverse events caused by IDH inhibitors. The above conclusions need more large samples and high-quality RCTs in the future to verify.
Keywords: Acute myeloid leukemia; Enasidenib; IDH inhibitors; Ivosidenib; Meta-analysis.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

