Activation of MAPK-mediated immunity by phosphatidic acid in response to positive-strand RNA viruses
- PMID: 37434356
- PMCID: PMC10811337
- DOI: 10.1016/j.xplc.2023.100659
Activation of MAPK-mediated immunity by phosphatidic acid in response to positive-strand RNA viruses
Abstract
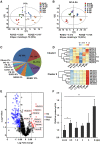
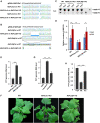
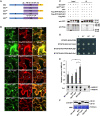
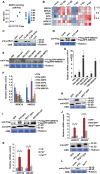
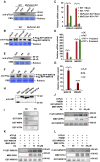
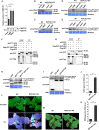
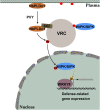
Increasing evidence suggests that mitogen-activated protein kinase (MAPK) cascades play a crucial role in plant defense against viruses. However, the mechanisms that underlie the activation of MAPK cascades in response to viral infection remain unclear. In this study, we discovered that phosphatidic acid (PA) represents a major class of lipids that respond to Potato virus Y (PVY) at an early stage of infection. We identified NbPLDα1 (Nicotiana benthamiana phospholipase Dα1) as the key enzyme responsible for increased PA levels during PVY infection and found that it plays an antiviral role. 6K2 of PVY interacts with NbPLDα1, leading to elevated PA levels. In addition, NbPLDα1 and PA are recruited by 6K2 to membrane-bound viral replication complexes. On the other hand, 6K2 also induces activation of the MAPK pathway, dependent on its interaction with NbPLDα1 and the derived PA. PA binds to WIPK/SIPK/NTF4, prompting their phosphorylation of WRKY8. Notably, spraying with exogenous PA is sufficient to activate the MAPK pathway. Knockdown of the MEK2-WIPK/SIPK-WRKY8 cascade resulted in enhanced accumulation of PVY genomic RNA. 6K2 of Turnip mosaic virus and p33 of Tomato bushy stunt virus also interacted with NbPLDα1 and induced the activation of MAPK-mediated immunity. Loss of function of NbPLDα1 inhibited virus-induced activation of MAPK cascades and promoted viral RNA accumulation. Thus, activation of MAPK-mediated immunity by NbPLDα1-derived PA is a common strategy employed by hosts to counteract positive-strand RNA virus infection.
Keywords: +RNA virus; MAPK; NbPLDα1; PA; PVY; Potato virus Y; WIPK/SIPK; phosphatidic acid; positive-strand RNA virus.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures
Similar articles
-
+RNA Viruses Enhance Plant Drought Tolerance Through Modulation of Phospholipase Dα1 (PLDα1)-Derived Phosphatidic Acid (PA).Plant Cell Environ. 2025 Sep;48(9):6552-6567. doi: 10.1111/pce.15637. Epub 2025 May 21. Plant Cell Environ. 2025. PMID: 40396382
-
Phosphorylation of the Nicotiana benthamiana WRKY8 transcription factor by MAPK functions in the defense response.Plant Cell. 2011 Mar;23(3):1153-70. doi: 10.1105/tpc.110.081794. Epub 2011 Mar 8. Plant Cell. 2011. PMID: 21386030 Free PMC article.
-
Identification of MAPKs as signal transduction components required for the cell death response during compatible infection by the synergistic pair Potato virus X-Potato virus Y.Virology. 2017 Sep;509:178-184. doi: 10.1016/j.virol.2017.06.022. Epub 2017 Jun 23. Virology. 2017. PMID: 28647505
-
The role of phosphatidic acid in the regulation of the Ras/MEK/Erk signaling cascade.FEBS Lett. 2002 Oct 30;531(1):65-8. doi: 10.1016/s0014-5793(02)03483-x. FEBS Lett. 2002. PMID: 12401205 Review.
-
Regulatory Mechanisms of Mitogen-Activated Protein Kinase Cascades in Plants: More than Sequential Phosphorylation.Int J Mol Sci. 2022 Mar 25;23(7):3572. doi: 10.3390/ijms23073572. Int J Mol Sci. 2022. PMID: 35408932 Free PMC article. Review.
Cited by
-
Phospholipid Signaling in Crop Plants: A Field to Explore.Plants (Basel). 2024 May 31;13(11):1532. doi: 10.3390/plants13111532. Plants (Basel). 2024. PMID: 38891340 Free PMC article.
-
A GDSL motif-containing lipase modulates Sclerotinia sclerotiorum resistance in Brassica napus.Plant Physiol. 2024 Dec 2;196(4):2973-2988. doi: 10.1093/plphys/kiae500. Plant Physiol. 2024. PMID: 39321167 Free PMC article.
References
-
- Andersson M.X., Kourtchenko O., Dangl J.L., Mackey D., Ellerström M. Phospholipase-dependent signalling during the AvrRpm1- and AvrRpt2-induced disease resistance responses in Arabidopsis thaliana. Plant J. 2006;47:947–959. - PubMed
-
- Asai T., Tena G., Plotnikova J., Willmann M.R., Chiu W.L., Gomez-Gomez L., Boller T., Ausubel F.M., Sheen J. MAP kinase signalling cascade in Arabidopsis innate immunity. Nature. 2002;415:977–983. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

