Temporal fingerprints of cortical gyrification in marmosets and humans
- PMID: 37434368
- PMCID: PMC10656951
- DOI: 10.1093/cercor/bhad245
Temporal fingerprints of cortical gyrification in marmosets and humans
Abstract
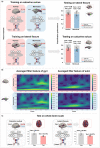
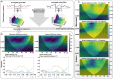
Recent neuroimaging studies in humans have reported distinct temporal dynamics of gyri and sulci, which may be associated with putative functions of cortical gyrification. However, the complex folding patterns of the human cortex make it difficult to explain temporal patterns of gyrification. In this study, we used the common marmoset as a simplified model to examine the temporal characteristics and compare them with the complex gyrification of humans. Using a brain-inspired deep neural network, we obtained reliable temporal-frequency fingerprints of gyri and sulci from the awake rs-fMRI data of marmosets and humans. Notably, the temporal fingerprints of one region successfully classified the gyrus/sulcus of another region in both marmosets and humans. Additionally, the temporal-frequency fingerprints were remarkably similar in both species. We then analyzed the resulting fingerprints in several domains and adopted the Wavelet Transform Coherence approach to characterize the gyro-sulcal coupling patterns. In both humans and marmosets, sulci exhibited higher frequency bands than gyri, and the two were temporally coupled within the same range of phase angles. This study supports the notion that gyri and sulci possess unique and evolutionarily conserved features that are consistent across functional areas, and advances our understanding of the functional role of cortical gyrification.
Keywords: Marmoset; convolutional neural network; cortical folding; functional connectivity; resting-state fMRI.
© The Author(s) 2023. Published by Oxford University Press. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
None declared.
Figures



Similar articles
-
Fetal sulcation and gyrification in common marmosets (Callithrix jacchus) obtained by ex vivo magnetic resonance imaging.Neuroscience. 2014 Jan 17;257:158-74. doi: 10.1016/j.neuroscience.2013.10.067. Epub 2013 Nov 9. Neuroscience. 2014. PMID: 24220690
-
Cortico-Subcortical Functional Connectivity Profiles of Resting-State Networks in Marmosets and Humans.J Neurosci. 2020 Nov 25;40(48):9236-9249. doi: 10.1523/JNEUROSCI.1984-20.2020. Epub 2020 Oct 23. J Neurosci. 2020. PMID: 33097633 Free PMC article.
-
Modeling functional difference between gyri and sulci within intrinsic connectivity networks.Cereb Cortex. 2023 Feb 7;33(4):933-947. doi: 10.1093/cercor/bhac111. Cereb Cortex. 2023. PMID: 35332916
-
Anatomical and functional neuroimaging in awake, behaving marmosets.Dev Neurobiol. 2017 Mar;77(3):373-389. doi: 10.1002/dneu.22456. Epub 2016 Oct 21. Dev Neurobiol. 2017. PMID: 27706916 Free PMC article. Review.
-
Neuroanatomic and magnetic resonance imaging references for normal development of cerebral sulci of laboratory primate, cynomolgus monkeys (Macaca fascicularis).Congenit Anom (Kyoto). 2012 Mar;52(1):16-27. doi: 10.1111/j.1741-4520.2011.00352.x. Congenit Anom (Kyoto). 2012. PMID: 22348780 Review.
Cited by
-
Diverse and asymmetric patterns of single-neuron projectome in regulating interhemispheric connectivity.Nat Commun. 2024 Apr 22;15(1):3403. doi: 10.1038/s41467-024-47762-y. Nat Commun. 2024. PMID: 38649683 Free PMC article.
References
-
- Belloy ME, Naeyaert M, Abbas A, Shah D, Vanreusel V, van Audekerke, Keilholz SD, Keliris GA, Van der Linden, Verhoye M. Dynamic resting state FMRI analysis in mice reveals a set of quasi-periodic patterns and illustrates their relationship with the global signal. NeuroImage Brain Connect Dyn. 2018:180(October):463–484. - PMC - PubMed
-
- Bolt T, Nomi JS, Bzdok D, Salas JA, Catie Chang BT, Yeo T, Uddin LQ, Keilholz SD. A parsimonious description of global functional brain organization in three spatiotemporal patterns. Nat Neurosci. 2022:25(8):1093–1103. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

