IFITM1 inhibits trophoblast invasion and is induced in placentas associated with IFN-mediated pregnancy diseases
- PMID: 37434700
- PMCID: PMC10331461
- DOI: 10.1016/j.isci.2023.107147
IFITM1 inhibits trophoblast invasion and is induced in placentas associated with IFN-mediated pregnancy diseases
Abstract
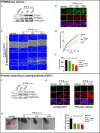
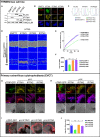
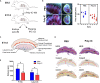
Interferon-induced transmembrane proteins (IFITMs) are restriction factors that block many viruses from entering cells. High levels of type I interferon (IFN) are associated with adverse pregnancy outcomes, and IFITMs have been shown to impair the formation of syncytiotrophoblast. Here, we examine whether IFITMs affect another critical step of placental development, extravillous cytotrophoblast (EVCT) invasion. We conducted experiments using in vitro/ex vivo models of EVCT, mice treated in vivo with the IFN-inducer poly (I:C), and human pathological placental sections. Cells treated with IFN-β demonstrated upregulation of IFITMs and reduced invasive abilities. Transduction experiments confirmed that IFITM1 contributed to the decreased cell invasion. Similarly, migration of trophoblast giant cells, the mouse equivalent of human EVCTs, was significantly reduced in poly (I:C)-treated mice. Finally, analysis of CMV- and bacterial-infected human placentas revealed upregulated IFITM1 expression. These data demonstrate that high levels of IFITM1 impair trophoblast invasion and could explain the placental dysfunctions associated with IFN-mediated disorders.
Keywords: Biological sciences; Cell biology; Virology.
© 2023 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Interferon-induced transmembrane proteins inhibit cell fusion mediated by trophoblast syncytins.J Biol Chem. 2019 Dec 27;294(52):19844-19851. doi: 10.1074/jbc.AC119.010611. Epub 2019 Nov 17. J Biol Chem. 2019. PMID: 31735710 Free PMC article.
-
Human chorionic gonadotropin produced by the invasive trophoblast but not the villous trophoblast promotes cell invasion and is down-regulated by peroxisome proliferator-activated receptor-gamma.Endocrinology. 2007 Oct;148(10):5011-9. doi: 10.1210/en.2007-0286. Epub 2007 Jul 12. Endocrinology. 2007. PMID: 17628005
-
Adrenomedullin enhances invasion of human extravillous cytotrophoblast-derived cell lines by regulation of urokinase plasminogen activator expression and s-nitrosylation.Biol Reprod. 2013 Feb 14;88(2):34. doi: 10.1095/biolreprod.112.103903. Print 2013 Feb. Biol Reprod. 2013. PMID: 23242529
-
PPARgamma and early human placental development.Curr Med Chem. 2008;15(28):3011-24. doi: 10.2174/092986708786848677. Curr Med Chem. 2008. PMID: 19075649 Review.
-
Role of nuclear receptors and their ligands in human trophoblast invasion.J Reprod Immunol. 2008 Apr;77(2):161-70. doi: 10.1016/j.jri.2007.05.004. Epub 2007 Aug 15. J Reprod Immunol. 2008. PMID: 17706792 Review.
Cited by
-
Endogenous retrovirus HERVH-derived lncRNA UCA1 controls human trophoblast development.Proc Natl Acad Sci U S A. 2024 Mar 19;121(12):e2318176121. doi: 10.1073/pnas.2318176121. Epub 2024 Mar 14. Proc Natl Acad Sci U S A. 2024. PMID: 38483994 Free PMC article.
-
Nucleotide metabolism, leukodystrophies, and CNS pathology.J Inherit Metab Dis. 2024 Sep;47(5):860-875. doi: 10.1002/jimd.12721. Epub 2024 Feb 29. J Inherit Metab Dis. 2024. PMID: 38421058 Review.
-
Hydroquinone impairs trophoblast migration and invasion via AHR-twist-IFITM1 axis.Placenta. 2024 Sep 26;155:88-99. doi: 10.1016/j.placenta.2024.07.315. Epub 2024 Aug 10. Placenta. 2024. PMID: 39173312
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

