Whole-body microbiota of newborn calves and their response to prenatal vitamin and mineral supplementation
- PMID: 37434710
- PMCID: PMC10331429
- DOI: 10.3389/fmicb.2023.1207601
Whole-body microbiota of newborn calves and their response to prenatal vitamin and mineral supplementation
Abstract
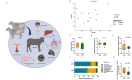
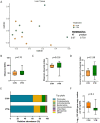
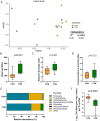
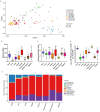
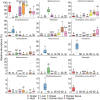
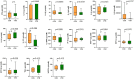
Early life microbial colonization and factors affecting colonization patterns are gaining interest due to recent developments suggesting that early life microbiome may play a role in Developmental Origins of Health and Disease. In cattle, limited information exists on the early microbial colonization of anatomical sites involved in bovine health beyond the gastrointestinal tract. Here, we investigated 1) the initial microbial colonization of seven different anatomical locations in newborn calves and 2) whether these early life microbial communities and 3) serum cytokine profiles are influenced by prenatal vitamin and mineral (VTM) supplementation. Samples were collected from the hoof, liver, lung, nasal cavity, eye, rumen (tissue and fluid), and vagina of beef calves that were born from dams that either received or did not receive VTM supplementation throughout gestation (n = 7/group). Calves were separated from dams immediately after birth and fed commercial colostrum and milk replacer until euthanasia at 30 h post-initial colostrum feeding. The microbiota of all samples was assessed using 16S rRNA gene sequencing and qPCR. Calf serum was subjected to multiplex quantification of 15 bovine cytokines and chemokines. Our results indicated that the hoof, eye, liver, lung, nasal cavity, and vagina of newborn calves were colonized by site-specific microbiota, whose community structure differed from the ruminal-associated communities (0.64 ≥ R2 ≥ 0.12, p ≤ 0.003). The ruminal fluid microbial community was the only one that differed by treatment (p < 0.01). However, differences (p < 0.05) by treatment were detected in microbial richness (vagina); diversity (ruminal tissue, fluid, and eye); composition at the phylum and genus level (ruminal tissue, fluid, and vagina); and in total bacterial abundance (eye and vagina). From serum cytokines evaluated, concentration of chemokine IP-10 was greater (p = 0.02) in VTM calves compared to control calves. Overall, our results suggest that upon birth, the whole-body of newborn calves are colonized by relatively rich, diverse, and site-specific bacterial communities. Noticeable differences were observed in ruminal, vaginal, and ocular microbiota of newborn calves in response to prenatal VTM supplementation. These findings can derive future hypotheses regarding the initial microbial colonization of different body sites, and on maternal micronutrient consumption as a factor that may influence early life microbial colonization.
Keywords: bovine; maternal; newborn; prenatal; vitamin and mineral supplementation; whole-body microbiota.
Copyright © 2023 Luecke, Holman, Schmidt, Gzyl, Hurlbert, Menezes, Bochantin, Kirsch, Baumgaertner, Sedivec, Swanson, Dahlen and Amat.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Effects of dietary restriction and one-carbon metabolite supplementation during the first 63 days of gestation on the maternal gut, vaginal, and blood microbiota in cattle.Anim Microbiome. 2024 Aug 29;6(1):48. doi: 10.1186/s42523-024-00335-2. Anim Microbiome. 2024. PMID: 39210404 Free PMC article.
-
Effects of antibiotic residues in milk on growth, ruminal fermentation, and microbial community of preweaning dairy calves.J Dairy Sci. 2019 Mar;102(3):2298-2307. doi: 10.3168/jds.2018-15506. Epub 2019 Jan 26. J Dairy Sci. 2019. PMID: 30692007
-
Early supplementation of Saccharomyces cerevisiae boulardii CNCM I-1079 in newborn dairy calves increases IgA production in the intestine at 1 week of age.J Dairy Sci. 2020 Sep;103(9):8615-8628. doi: 10.3168/jds.2020-18274. Epub 2020 Jul 16. J Dairy Sci. 2020. PMID: 32684462
-
A 100-Year Review: Calf nutrition and management.J Dairy Sci. 2017 Dec;100(12):10151-10172. doi: 10.3168/jds.2017-13062. J Dairy Sci. 2017. PMID: 29153160 Review.
-
Invited review: Impact of maternal health and nutrition on the microbiome and immune development of neonatal calves.J Dairy Sci. 2024 Oct;107(10):7504-7519. doi: 10.3168/jds.2024-24835. Epub 2024 May 31. J Dairy Sci. 2024. PMID: 38825126 Review.
Cited by
-
Maternal Overnutrition in Beef Cattle: Effects on Fetal Programming, Metabolic Health, and Postnatal Outcomes.Biology (Basel). 2025 Jun 2;14(6):645. doi: 10.3390/biology14060645. Biology (Basel). 2025. PMID: 40563896 Free PMC article. Review.
-
Combined analysis of 16S rRNA gene sequencing data reveals core vaginal bacteria across livestock species.Front Microbiol. 2025 Feb 10;16:1524000. doi: 10.3389/fmicb.2025.1524000. eCollection 2025. Front Microbiol. 2025. PMID: 39996073 Free PMC article.
-
Contribution of the seminal microbiome to paternal programming.Biol Reprod. 2024 Aug 15;111(2):242-268. doi: 10.1093/biolre/ioae068. Biol Reprod. 2024. PMID: 38696371 Free PMC article. Review.
-
Holistic View and Novel Perspective on Ruminal and Extra-Gastrointestinal Methanogens in Cattle.Microorganisms. 2023 Nov 10;11(11):2746. doi: 10.3390/microorganisms11112746. Microorganisms. 2023. PMID: 38004757 Free PMC article. Review.
-
A Comprehensive Review of Bovine Colostrum Components and Selected Aspects Regarding Their Impact on Neonatal Calf Physiology.Animals (Basel). 2024 Apr 8;14(7):1130. doi: 10.3390/ani14071130. Animals (Basel). 2024. PMID: 38612369 Free PMC article. Review.
References
-
- Amachawadi R. G., Tom W. A., Hays M. P., Fernando S. C., Hardwidge P. R., Nagaraja T. G. (2021). Bacterial community analysis of purulent material from liver abscesses of crossbred cattle and Holstein steers fed finishing diets with or without tylosin. J. Anim. Sci. 99:skab076. doi: 10.1093/jas/skab076, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

