Chemo-enzymatic synthesis and biological activity evaluation of propenylbenzene derivatives
- PMID: 37434714
- PMCID: PMC10330721
- DOI: 10.3389/fmicb.2023.1223123
Chemo-enzymatic synthesis and biological activity evaluation of propenylbenzene derivatives
Abstract
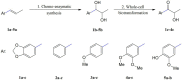
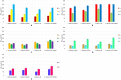
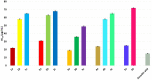
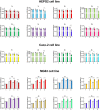
Propenylbenzenes, including isosafrole, anethole, isoeugenol, and their derivatives, are natural compounds found in essential oils from various plants. Compounds of this group are important and valuable, and are used in the flavour and fragrance industries as well as the pharmaceutical and cosmetic industries. The aim of this study was to develop an efficient process for synthesising oxygenated derivatives of these compounds and evaluate their potential biological activities. In this paper, we propose a two-step chemo-enzymatic method. The first step involves the synthesis of corresponding diols 1b-5b from propenylbenzenes 1a-5avia lipase catalysed epoxidation followed by epoxide hydrolysis. The second step involves the microbial oxidation of a diasteroisomeric mixture of diols 1b-5b to yield the corresponding hydroxy ketones 1c-4c, which in this study was performed on a preparative scale using Dietzia sp. DSM44016, Rhodococcus erythropolis DSM44534, R. erythropolis PCM2150, and Rhodococcus ruber PCM2166. Application of scaled-up processes allowed to obtain hydroxy ketones 1-4c with the following yield range 36-62.5%. The propenylbenzene derivatives thus obtained and the starting compounds were tested for various biological activities, including antimicrobial, antioxidant, haemolytic, and anticancer activities, and their impact on membrane fluidity. Fungistatic activity assay against selected strains of Candida albicans results in MIC50 value varied from 37 to 124 μg/mL for compounds 1a, 3a-c, 4a,b, and 5a,b. The highest antiradical activity was shown by propenylbenzenes 1-5a with a double bond in their structure with EC50 value ranged from 19 to 31 μg/mL. Haemolytic activity assay showed no cytotoxicity of the tested compounds on human RBCs whereas, compounds 2b-4b and 2c-4c affected the fluidity of the RBCs membrane. The tested compounds depending on their concentration showed different antiproliferative activity against HepG2, Caco-2, and MG63. The results indicate the potential utility of these compounds as fungistatics, antioxidants, and proliferation inhibitors of selected cell lines.
Keywords: antioxidant activity; biotransformation; fragrances; fungistatic activity; haemolytic activity; oxidation; proliferative activity; propenylbenzenes.
Copyright © 2023 Hernik, Szczepańska, Ghezzi, Brenna, Włoch, Pruchnik, Mularczyk, Marycz, Olejniczak and Boratyński.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Stereoselective synthesis of whisky lactone isomers catalyzed by bacteria in the genus Rhodococcus.Front Microbiol. 2023 Jan 19;14:1117835. doi: 10.3389/fmicb.2023.1117835. eCollection 2023. Front Microbiol. 2023. PMID: 36744099 Free PMC article.
-
Biotransformations of propenylbenzenes by an Arthrobacter sp. and its t-anethole blocked mutants.J Biotechnol. 2003 Oct 9;105(1-2):61-70. doi: 10.1016/s0168-1656(03)00141-x. J Biotechnol. 2003. PMID: 14511910
-
Bacterial Biotransformation of Oleic Acid: New Findings on the Formation of γ-Dodecalactone and 10-Ketostearic Acid in the Culture of Micrococcus luteus.Molecules. 2020 Jul 2;25(13):3024. doi: 10.3390/molecules25133024. Molecules. 2020. PMID: 32630666 Free PMC article.
-
Microbial transformation of propenylbenzenes for natural flavour production.Trends Biotechnol. 2007 Dec;25(12):571-6. doi: 10.1016/j.tibtech.2007.08.011. Epub 2007 Nov 7. Trends Biotechnol. 2007. PMID: 17988755 Review.
-
Cephalostatin analogues--synthesis and biological activity.Fortschr Chem Org Naturst. 2004;87:1-80. doi: 10.1007/978-3-7091-0581-8_1. Fortschr Chem Org Naturst. 2004. PMID: 15079895 Review.
References
-
- Björkling F., Godtfredsen S. E., Kik O. (1990). Lipase-mediated formation of peroxycarboxylic acids used in catalytic epoxidation of alkenes. J. Chem. Soc. Chem. Commun. 19, 1301–1303. doi: 10.1039/C39900001301 - DOI
-
- Bruna F., Fernandez K., Urrejola F., Touma J., Navarro M., Sepulveda B., et al. . (2022). Chemical composition, antioxidant, antimicrobial and antiproliferative activity of Laureliopsis philippiana essential oil of Chile, study in vitro and in silico. Arab. J. Chem. 15:104271. doi: 10.1016/j.arabjc.2022.104271 - DOI
LinkOut - more resources
Full Text Sources

