Facile Titrimetric Assay of Lysophosphatidic Acid in Human Serum and Plasma for Ovarian Cancer Detection
- PMID: 37434795
- PMCID: PMC10331031
- DOI: 10.15430/JCP.2023.28.2.31
Facile Titrimetric Assay of Lysophosphatidic Acid in Human Serum and Plasma for Ovarian Cancer Detection
Abstract
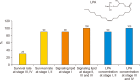
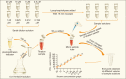
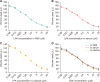
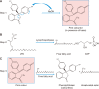
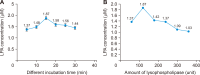
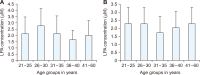
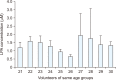
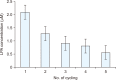
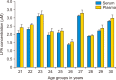
Herein, an instrument free facile acid-base titrimetric methodology is reported for lysophosphatidic acid (LPA) measurement in serum and plasma samples for ovarian cancer detection. The concept is based on the titrimetric method in which alkaline solution was titrated with free fatty acid. Free fatty acid is generated due to action of the lysophospholipase to LPA. A phospholipid derivative known as LPA can function as a signaling molecule. A glycerol backbone serves as the foundation for phosphatidic acid, which also has bonds to an unsaturated fatty acid at carbon-1, a hydroxyl group at carbon-2, and a phosphate molecule at carbon-3. Free fatty acid and glycerol-3-phosphate are formed when LPA reacts with lysophospholipase. The formation of free fatty acid depends on the concentration of LPA. The standard graph of known concentrations of LPA, LPA spiked serum and LPA spiked plasma was plotted. The concentration of LPA in unknown serum and plasma were calculated from the standard graph. The limit of detection of LPA in spiked serum and plasma samples via titrimetric assay was calculated as 0.156 μmol/L. A patient's chance of survival may be outweighed by an early diagnosis of ovarian cancer.
Keywords: Acid-base; Assay; Concentration; Lysophosphatidic acid; Titration.
Copyright © 2023 Korean Society of Cancer Prevention.
Conflict of interest statement
CONFLICTS OF INTEREST No potential conflicts of interest were disclosed.
Figures
Similar articles
-
A novel colorimetric assay for the determination of lysophosphatidic acid in plasma using an enzymatic cycling method.Clin Chim Acta. 2003 Jul 1;333(1):59-67. doi: 10.1016/s0009-8981(03)00165-7. Clin Chim Acta. 2003. PMID: 12809736
-
Lack of significant differences in the corrected activity of lysophospholipase D, producer of phospholipid mediator lysophosphatidic acid, in incubated serum from women with and without ovarian tumors.Cancer. 2002 Jan 1;94(1):141-51. doi: 10.1002/cncr.10146. Cancer. 2002. PMID: 11815970
-
Fatty acid composition of lysophosphatidic acid and lysophosphatidylinositol in plasma from patients with ovarian cancer and other gynecological diseases.Gynecol Oncol. 2001 Oct;83(1):25-30. doi: 10.1006/gyno.2001.6357. Gynecol Oncol. 2001. PMID: 11585410
-
Physiological and pathophysiological roles of lysophosphatidic acids produced by secretory lysophospholipase D in body fluids.Biochim Biophys Acta. 2002 May 23;1582(1-3):18-25. doi: 10.1016/s1388-1981(02)00133-6. Biochim Biophys Acta. 2002. PMID: 12069806 Review.
-
Lysophosphatidic acid, a simple phospholipid with myriad functions.Pharmacol Ther. 2023 Jun;246:108421. doi: 10.1016/j.pharmthera.2023.108421. Epub 2023 Apr 18. Pharmacol Ther. 2023. PMID: 37080433 Review.
References
-
- Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C. GLOBOCAN 2012: Estimated cancer incidence, mortality and prevalence worldwide in 2012. [Accessed February 14, 2020]. https://www.scienceopen.com/document?vid=19f26dca-3511-48bf-8fab-76242d4....
LinkOut - more resources
Full Text Sources
Miscellaneous
