A meta-analysis of the incidence and risk of skin toxicity with nab-paclitaxel and paclitaxel in cancer treatment
- PMID: 37434856
- PMCID: PMC10331690
A meta-analysis of the incidence and risk of skin toxicity with nab-paclitaxel and paclitaxel in cancer treatment
Abstract
Background: Skin toxicity of varying severity occurs mostly during various courses of chemotherapy. In clinical trials and practice, we have found that both nab-paclitaxel and paclitaxel cause side effects such as rash and pruritus. To further clarify the incidence of rash and pruritus in both, we conducted the present study by a systematic evaluation, the results of which can be used to guide clinical dosing choices.
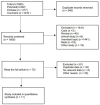
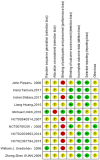
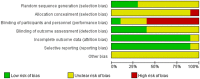
Methods: An electrical search was performed on randomized controlled research trials of nab-paclitaxel and paclitaxel for the treatment of malignancies. The necessary data were extracted, integrated, and analyzed from the included studies by systematic evaluation and meta-analysis, depending on the study design. Further subgroup analyses were performed to explore the incidence of rash and pruritus in nab-paclitaxel and paclitaxel.
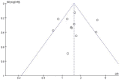
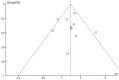
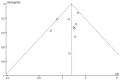
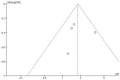
Results: Eleven studies with a total of 971 patients with malignancy were included. Four studies were application of single-agent nab-paclitaxel compared with paclitaxel, and seven studies were comparative chemotherapy drug combinations. The incidence of rash was higher in all grades of nab-paclitaxel than that in paclitaxel (OR=1.39, CI 95% [1.18-1.62]); the incidence of rash was higher in lower grades of paclitaxel than that in solvent-based paclitaxel (OR=1.31, CI 95% [1.11-1.53]); the incidence of rash was higher in all grades in the single-agent application comparison. The incidence of rash was higher in nab-paclitaxel than that in paclitaxel (OR=1.81, CI 95% [1.26-2.59]); there was no significant difference in the incidence of pruritus between nab-paclitaxel and paclitaxel (OR=1.19, CI 95% [0.88-1.61]).
Conclusion: In comparison with paclitaxel, nab-paclitaxel significantly increased the risk of a teething rash. There was a significant risk correlation between nab-paclitaxel and teething rash. Early prevention, identification, and treatment of rash could significantly improve patient's quality of life and optimize their clinical survival.
Keywords: META analysis; Paclitaxel; nab-paclitaxel; pruritus; rash.
AJTR Copyright © 2023.
Conflict of interest statement
None.
Figures



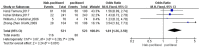


References
-
- Erratum: global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2020;70:313. - PubMed
-
- Rosenberg SM, O’Neill A, Sepucha K, Miller KD, Dang CT, Northfelt DW, Sledge GW, Schneider BP, Partridge AH. Quality of life following receipt of adjuvant chemotherapy with and without bevacizumab in patients with lymph node-positive and high-risk lymph node-negative breast cancer. JAMA Netw Open. 2022;5:e220254. - PMC - PubMed
-
- Sugawara S, Lee JS, Kang JH, Kim HR, Inui N, Hida T, Lee KH, Yoshida T, Tanaka H, Yang CT, Nishio M, Ohe Y, Tamura T, Yamamoto N, Yu CJ, Akamatsu H, Namba Y, Sumiyoshi N, Nakagawa K. Nivolumab with carboplatin, paclitaxel, and bevacizumab for first-line treatment of advanced nonsquamous non-small-cell lung cancer. Ann Oncol. 2021;32:1137–1147. - PubMed
-
- van der Heijden MS, Loriot Y, Durán I, Ravaud A, Retz M, Vogelzang NJ, Nelson B, Wang J, Shen X, Powles T. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma: a long-term overall survival and safety update from the phase 3 IMvigor211 clinical trial. Eur Urol. 2021;80:7–11. - PubMed
-
- Tsuburaya A, Yoshida K, Kobayashi M, Yoshino S, Takahashi M, Takiguchi N, Tanabe K, Takahashi N, Imamura H, Tatsumoto N, Hara A, Nishikawa K, Fukushima R, Nozaki I, Kojima H, Miyashita Y, Oba K, Buyse M, Morita S, Sakamoto J. Sequential paclitaxel followed by tegafur and uracil (UFT) or S-1 versus UFT or S-1 monotherapy as adjuvant chemotherapy for T4a/b gastric cancer (SAMIT): a phase 3 factorial randomised controlled trial. Lancet Oncol. 2014;15:886–93. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
