The German CaRe high registry for familial hypercholesterolemia - Sex differences, treatment strategies, and target value attainment
- PMID: 37434912
- PMCID: PMC10331285
- DOI: 10.1016/j.athplu.2023.06.001
The German CaRe high registry for familial hypercholesterolemia - Sex differences, treatment strategies, and target value attainment
Abstract
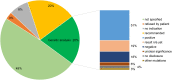
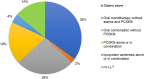
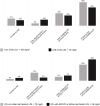
Background and aims: Familial hypercholesterolemia (FH) is among the most common genetic disorders in primary care. However, only 15% or less of patients are diagnosed, and few achieve the goals for low-density lipoprotein cholesterol (LDL-C). In this analysis of the German Cascade Screening and Registry for High Cholesterol (CaRe High), we examined the status of lipid management, treatment strategies, and LDL-C goal attainment according to the ESC/EAS dyslipidemia guidelines.
Methods: We evaluated consolidated datasets from 1501 FH patients diagnosed clinically and seen either by lipid specialists or general practitioners and internists. We conducted a questionnaire survey of both the recruiting physicians and patients.
Results: Among the 1501 patients, 86% regularly received lipid-lowering drugs. LDL-C goals were achieved by 26% and 10% of patients with atherosclerotic cardiovascular disease (ASCVD) according to the 2016 and 2019 ESC/EAS dyslipidemia guidelines, respectively. High intensity lipid-lowering was administered more often in men than in women, in patients with ASCVD, at higher LDL-C and in patients with a genetic diagnosis of FH.
Conclusions: FH is under-treated in Germany compared to guideline recommendations. Male gender, genetic proof of FH, treatment by a specialist, and presence of ASCVD appear to be associated with increased treatment intensity. Achieving the LDL-C goals of the 2019 ESC/EAS dyslipidemia guidelines remains challenging if pre-treatment LDL-C is very high.
Keywords: Adherence; Cardiovascular risk; Cascade screening; Familial hypercholesterolemia; Low-density lipoproteins; Patient registry; Treatment.
© 2023 The Authors.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: NS was project lead of the CaRe High registry, which is financed in cooperation with Amgen GmbH and Sanofi-Aventis GmbH. IaH reports lecture fees from Synlab. NS reports non-financial support from Amgen GmbH during the conduct of the study. AD is employed by the CaRe High registry. TBG, AB, ACK, WR, CK, MM, KSJ, JLK, and CBO have nothing to disclose. IGB has received honoraria for consulting and/or lecture fees from Amgen, Sanofi-Aventis, Aegereon, Regeneron, Novartis, Pfizer, Synlab, Akcea Therapeutics, and Daiichi-Sankyo and institutional support from Amgen, Sanofi-Aventis, Akcea Therapeutics, DACH, and Novartis. HH reports lecture fees from Amgen, Berlin-Chemie, Daiichi-Sankio, Pfizer, and Sanofi-Aventis. SK received honoraria as a speaker and member of advisory boards from Amgen, AstraZeneca, Berlin Chemie, Boehringer Ingelheim, Daiichi Sankyo, Medical Association North Rhine Germany, Novartis, Roche, Sanofi-Aventis, and Servier. FUB reports lecture fees from Amgen, Sanofi-Aventis, and Synlab outside the submitted work. UK reports grants from D.A.C.H. Society for Prevention of cardiovascular disease during the conduct of the study; others from Amgen, Sanofi-Aventis, Alexion, Berlin Chemie, and Fresenius Medical Care outside the submitted work. GK reports lecture/advisory board fees from Akcea, Amgen, BMS, MSD, Sanofi-Aventis, and Synlab outside the submitted work. UJ reports honoraria from Aegerion, Akcea, Amgen, Chiesi, Sanofi-Aventis, Kaneka, Diamed, and Fresenius Medical Care outside the submitted work. WK reports personal fees from AstraZeneca, Novartis, Pfizer, The Medicines Company, DalCor, Kowa, Amgen, Corvidia, Berlin-Chemie, and Sanofi-Aventis, and grants and non-financial support from Beckmann, Singulex, Abbott, and Roche Diagnostics outside the submitted work; BO reports lecture/advisory board fees from Amgen, Amryt, Berlin Chemie, Hexal, MSD, and Sanofi-Aventis outside the submitted work. UL reports personal honoraria for lecture fees and other from Amgen, BerlinChemie, MSD, and Sanofi-Aventis outside the submitted work. EST received speakers' honoraria for presentations, advisory board activities, and funding of research projects by Fresenius Medical Care Germany, Amgen, Sanofi-Aventis, and Berlin Chemie. HS reports personal fees from AMGEN, Sanofi-Aventis, and MSD SHARP&DOHME outside the submitted work. KP reports personal fees from Akcea, Amarin, Amgen, Berlin-Chemie, Boehringer-Ingelheim, MSD, Daiichi-Sankyo, and Sanofi-Aventis outside the submitted work. AV reports lecture/advisory board fees from Aegerion, Amgen, Berlin Chemie, Fresenius, MSD, Regeneron/Sanofi-Aventis, and Synlab outside the submitted work. DMW received honoraria as speaker and member of advisory boards from Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, MSD, Novo Nordisk, Novartis, Sanofi-Aventis, and Sanofi-Pasteur. US reports honoraria for consultancy and lecturing from Amgen, Amarin, AstraZeneca, Berlin Chemie, Boehringer Ingelheim, Daiichi Sankyo, MSD, Sanofi-Aventis, Synlab, Novartis, and NovoNordisk.WM reports grants and personal fees from Sanofi-Aventis during the conduct of the study; grants from Siemens Healthineers, Astra-Zeneca, Bayer Vital GmbH, bestbion dx GmbH, Boehringer Ingelheim Pharma GmbH Co KG, Immundiagnostik GmbH, Merck Chemicals GmbH, MSD Sharp and Dohme GmbH, Novartis Pharma GmbH, and Olink Proteomics, grants and personal fees from Aegerion Pharmaceuticals, AMGEN, Alexion Pharmaceuticals, BASF, Abbott Diagnostics, Numares AG, Berlin-Chemie, and Akzea Therapeutics, and other from Synlab Holding Deutschland GmbH outside the submitted work.
Figures

Similar articles
-
Lipid-modifying therapy and low-density lipoprotein cholesterol goal attainment in patients with familial hypercholesterolemia in Germany: The CaReHigh Registry.Atherosclerosis. 2018 Oct;277:314-322. doi: 10.1016/j.atherosclerosis.2018.08.050. Atherosclerosis. 2018. PMID: 30270065
-
LDL cholesterol target achievement in heterozygous familial hypercholesterolemia patients according to 2019 ESC/EAS lipid guidelines: Implications for newer lipid-lowering treatments.Int J Cardiol. 2021 Dec 15;345:119-124. doi: 10.1016/j.ijcard.2021.10.024. Epub 2021 Oct 21. Int J Cardiol. 2021. PMID: 34687802
-
Low-density lipoprotein apheresis: an evidence-based analysis.Ont Health Technol Assess Ser. 2007;7(5):1-101. Epub 2006 Nov 1. Ont Health Technol Assess Ser. 2007. PMID: 23074505 Free PMC article.
-
Genetic testing for familial hypercholesterolemia: Impact on diagnosis, treatment and cardiovascular risk.Eur J Prev Cardiol. 2019 Aug;26(12):1262-1270. doi: 10.1177/2047487319829746. Epub 2019 Feb 12. Eur J Prev Cardiol. 2019. PMID: 30755017
-
Familial Hypercholesterolemia: Global Burden and Approaches.Curr Cardiol Rep. 2021 Sep 4;23(10):151. doi: 10.1007/s11886-021-01565-5. Curr Cardiol Rep. 2021. PMID: 34480646 Review.
Cited by
-
Lipoprotein Metabolism, Dyslipidemia, and Lipid-Lowering Therapy in Women: A Comprehensive Review.Pharmaceuticals (Basel). 2024 Jul 9;17(7):913. doi: 10.3390/ph17070913. Pharmaceuticals (Basel). 2024. PMID: 39065763 Free PMC article. Review.
-
Lipid Lowering Therapy Utilization and Lipid Goal Attainment in Women.Curr Atheroscler Rep. 2025 Jan 28;27(1):29. doi: 10.1007/s11883-025-01275-1. Curr Atheroscler Rep. 2025. PMID: 39873822 Free PMC article. Review.
References
-
- Brænne I., Kleinecke M., Reiz B., Graf E., Strom T., Wieland T., Fischer M., Kessler T., Hengstenberg C., Meitinger T., Erdmann J., Schunkert H. Systematic analysis of variants related to familial hypercholesterolemia in families with premature myocardial infarction. Eur J Hum Genet. 2016;24:191–197. doi: 10.1038/ejhg.2015.100. - DOI - PMC - PubMed
-
- Goldberg A.C., Hopkins P.N., Toth P.P., Ballantyne C.M., Rader D.J., Robinson J.G., Daniels S.R., Gidding S.S., De Ferranti S.D., Ito M.K., McGowan M.P., Moriarty P.M., Cromwell W.C., Ross J.L., Ziajka P.E. Familial hypercholesterolemia: screening, diagnosis and management of pediatric and adult patients: clinical guidance from the national lipid association expert panel on familial hypercholesterolemia. J. Clin. Lipidol. 2011:133–140. doi: 10.1016/j.jacl.2011.03.001. - DOI - PubMed
-
- Abifadel M., Varret M., Rabès J.P., Allard D., Ouguerram K., Devillers M., Cruaud C., Benjannet S., Wickham L., Erlich D., Derré A., Villéger L., Farnier M., Beucler I., Bruckert E., Chambaz J., Chanu B., Lecerf J.M., Luc G., Moulin P., Weissenbach J., Prat A., Krempf M., Junien C., Seidah N.G., Boileau C. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet. 2003 doi: 10.1038/ng1161. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
