Lack of Clinical Control in COPD Patients Depending on the Target and the Therapeutic Option
- PMID: 37434953
- PMCID: PMC10332360
- DOI: 10.2147/COPD.S414910
Lack of Clinical Control in COPD Patients Depending on the Target and the Therapeutic Option
Abstract
Introduction: According to the Global Initiative for chronic obstructive lung disease (GOLD), when a treatment is not achieving an appropriate response it should be switched taking into account the predominant treatable trait to target (dyspnea or exacerbations). The objective of the present study was to investigate the lack of clinical control according to the target and medication groups.
Materials and methods: This was a post-hoc analysis of the CLAVE study, an observational, cross-sectional, multicenter study which evaluated the clinical control, and related-factors, in a cohort of 4801 patients with severe chronic obstructive pulmonary disease (COPD). The primary endpoint was the percentage of uncontrolled patients defined as COPD Assessment Test (CAT) >16 or presence of exacerbations in the last 3 months despite receiving long-acting beta2-agonist (LABA) and/or long-acting antimuscarinic antagonist (LAMA) with or without inhaled corticosteroids (ICS). Secondary objectives included the description of sociodemographic and clinical characteristics of patients by therapeutic group and the identification of characteristics potentially associated with the lack of control of COPD including low adherence measured by the test to adherence to inhalers (TAI).
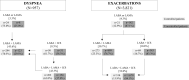
Results: In the dyspnea pathway, lack of clinical control was of 25.0% of patients receiving LABA or LAMA in monotherapy, 29.5% by those with LABA + LAMA, 38.3% with LABA + ICS and 37.0% with triple therapy (LABA + LAMA + ICS). In the exacerbation pathway, percentages were 87.1%, 76.7%, 83.3%, and 84.1%, respectively. Low physical activity and high Charlson comorbidity index were independent factor of non-control in all therapeutic groups. Additional factors were lower post-bronchodilator FEV1 and poor adherence to inhalers.
Conclusion: There are still room for improvement in COPD control. From the pharmacological perspective, every step in treatment have a pool of uncontrolled patients in which a step-up could be considered according to a trait to target strategy.
Keywords: COPD; comorbidities; control; dyspnea; target; treatment.
© 2023 Soler-Cataluña et al.
Conflict of interest statement
Pere Almagro declares he has received speaking or advisory fees, or economic aid to attend congresses from Astra-Zeneca, GSK, Novartis, Chiesi, Menarini, Boehringer-Ingelheim, Ferrer, and Rovi. Borja G Cosío declares he has received speaking or advisory fees, or economic aid to attend congresses from Astra-Zeneca, GSK, Novartis, Chiesi, Mundipharma, Menarini, Sanofi, TEVA, Boehringer-Ingelheim, and Rovi. He also reports non-financial support from Separ. Juan José Soler-Cataluña has received speaker fees from AstraZeneca, Bial, Boehringer Ingelheim, Chiesi, FAES, GSK, Menarini, and consulting fees from Bial, Boehringer Ingelheim, Chiesi and GSK. Diego Gonzalez-Segura is an employee of Chiesi España SAU, the sponsor of the study. The authors report no other conflicts of interest in this work.
Figures
References
-
- Ur Rehman A, Hassali MAA, Abbas S, et al. Pharmacological and non-pharmacological management of COPD; limitations and future prospects: a review of current literature. J Public Health. 2020;28(4):357–366. doi:10.1007/s10389-019-01021-3 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

