TP53-Mutated Myelodysplasia and Acute Myeloid Leukemia
- PMID: 37435040
- PMCID: PMC10332352
- DOI: 10.4084/MJHID.2023.038
TP53-Mutated Myelodysplasia and Acute Myeloid Leukemia
Abstract
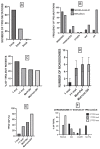
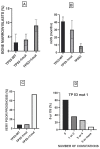
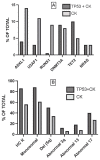
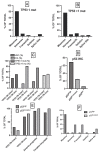
TP53-mutated myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) form a distinct and heterogeneous group of myeloid malignancies associated with poor outcomes. Studies carried out in the last years have in part elucidated the complex role played by TP53 mutations in the pathogenesis of these myeloid disorders and in the mechanisms of drug resistance. A consistent number of studies has shown that some molecular parameters, such as the presence of a single or multiple TP53 mutations, the presence of concomitant TP53 deletions, the association with co-occurring mutations, the clonal size of TP53 mutations, the involvement of a single (monoallelic) or of both TP53 alleles (biallelic) and the cytogenetic architecture of concomitant chromosome abnormalities are major determinants of outcomes of patients. The limited response of these patients to standard treatments, including induction chemotherapy, hypomethylating agents and venetoclax-based therapies and the discovery of an immune dysregulation have induced a shift to new emerging therapies, some of which being associated with promising efficacy. The main aim of these novel immune and nonimmune strategies consists in improving survival and in increasing the number of TP53-mutated MDS/AML patients in remission amenable to allogeneic stem cell transplantation.
Keywords: Acute myeloid leukemias; Cytogenetic characterization; Gene sequencing; Molecular abnormalities; Myelodysplastic syndromes; TP53.
Conflict of interest statement
Competing interests: The authors declare no conflict of Interest.
Figures

References
-
- Roman E, Smith A, Appleton S, Crouch S, Kelly R, Kinsey S, Corgo C, Patmore R. Myeloid malignancies in the real-world: occurrence, progression and survival in the UK’s population-based Haematological Malignancy Research Network 20094–15. Cancer Epidemiol. 2016;42:186–198. doi: 10.1016/j.canep.2016.03.011. - DOI - PMC - PubMed
-
- Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitrz MT, Porwit A, Harris NL, LeBeau MM, Hallstrom-Lindberg E, Tefferi A, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114:937–951. doi: 10.1182/blood-2009-03-209262. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

