Recombinant proteins A29L, M1R, A35R, and B6R vaccination protects mice from mpox virus challenge
- PMID: 37435062
- PMCID: PMC10331816
- DOI: 10.3389/fimmu.2023.1203410
Recombinant proteins A29L, M1R, A35R, and B6R vaccination protects mice from mpox virus challenge
Abstract
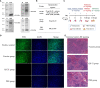
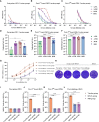
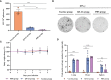
Since May 2022, mutant strains of mpox (formerly monkeypox) virus (MPXV) have been rapidly spreading among individuals who have not traveled to endemic areas in multiple locations, including Europe and the United States. Both intracellular and extracellular forms of mpox virus have multiple outer membrane proteins that can stimulate immune response. Here, we investigated the immunogenicity of MPXV structural proteins such as A29L, M1R, A35R, and B6R as a combination vaccine, and the protective effect against the 2022 mpox mutant strain was also evaluated in BALB/c mice. After mixed 15 μg QS-21 adjuvant, all four virus structural proteins were administered subcutaneously to mice. Antibody titers in mouse sera rose sharply after the initial boost, along with an increased capacity of immune cells to produce IFN-γ alongside an elevated level of cellular immunity mediated by Th1 cells. The vaccine-induced neutralizing antibodies significantly inhibited the replication of MPXV in mice and reduced the pathological damage of organs. This study demonstrates the feasibility of a multiple recombinant vaccine for MPXV variant strains.
Keywords: antibody response; mpox virus; recombinant protein; vaccination; virus challenge.
Copyright © 2023 Tang, Liu, Lu, Fan, Xu, Sun, Wang, Li, Dan, Du, Wang, Li and Yang.
Conflict of interest statement
All authors were employed by the company Wuhan Institute of Biological Products Co., Ltd.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

