Efficacy and safety of first-line immunotherapy plus chemotherapy in treating patients with extensive-stage small cell lung cancer: a Bayesian network meta-analysis
- PMID: 37435087
- PMCID: PMC10331819
- DOI: 10.3389/fimmu.2023.1197044
Efficacy and safety of first-line immunotherapy plus chemotherapy in treating patients with extensive-stage small cell lung cancer: a Bayesian network meta-analysis
Abstract
Background: Despite numerous immunotherapy and chemotherapy regimens available for patients with extensive-stage small cell lung cancer (ES-SCLC), it remains unclear which regimen is the most effective and safest; relative studies comparing such regimens are scarce.
Objective: The aim of this study was to investigate the efficacy and safety of first-line immunotherapy combinations with chemotherapy for patients with extensive-stage small cell lung cancer. In addition, for the first time, comparisons among the first-line systemic regimens on OS and PFS in ES-SCLC by each time node were made.
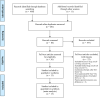
Methods: Databases including PubMed, Embase, Cochrane Library, Scopus, Google Scholars, and ClinicalTrials.gov, and major international conferences were searched for randomized controlled trials (RCTs) regarding comparing immunotherapy combinations with chemotherapy as first-line treatments for patients with advanced ES-SCLC from inception to 1 November. Hazard ratios (HRs) and odds ratios (ORs) were generated for dichotomous variants by RStudio 4.2.1. The outcomes comprised overall survival (OS), progression-free survival (PFS), objective response rate (ORR), and adverse events of grade 3 or higher (Grade ≥ 3 AEs).
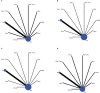
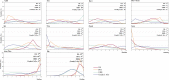
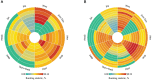
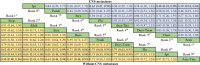
Results: Eventually, a total of nine RCTs reporting 4,352 individuals with nine regimens were enrolled. The regimens were ipilimumabnu (Ipi), atezolizumab (Atez), durvalumab plus tremelimumab (Durv-Trem), durvalumab (Durv), pembrolizumab (Pemb), adebrelimab (Adeb), serplulimab (Serp), atezolizumab plus tiragolumab (Atez-Tira), and nivolumab (Nivo). With regard to OS, serplulimab (HR = 0.63, 95% CI: 0.49 to 0.81) was found to yield the best OS benefit when compared with chemotherapy. Meanwhile, serplulimab had the highest probability (46.11%) for better OS. Furthermore, compared with chemotherapy, serplulimab significantly increased the OS rate from the 6th to the 21st month. With regard to PFS, serplulimab (HR = 0.47, 95% CI: 0.38 to 0.59) was found to yield the best PFS benefit when compared with chemotherapy. Simultaneously, serplulimab had the highest probability (94.48%) for better PFS. Serplulimab was also a long-lasting first-line regimen in both OS and PFS from a longitudinal perspective. In addition, there was no significant difference among the various treatment options for ORR and grade ≥3 AEs.
Conclusion: Considering OS, PFS, ORR, and safety profiles, serplulimab with chemotherapy should be recommended as the best therapy for patients with ES-SCLC. Certainly, more head-to-head studies are needed to confirm these findings.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/, identifier CRD42022373291.
Keywords: efficacy; extensive-stage small cell lung cancer; immunotherapy; network meta-analysis; safety.
Copyright © 2023 Zhang, Li, Diwu, Chen, Chen and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Efficacy and safety of different immunotherapies combined with chemotherapy as first-line therapy in patients with small cell lung cancer: a network meta-analysis.Front Immunol. 2024 Apr 17;15:1362537. doi: 10.3389/fimmu.2024.1362537. eCollection 2024. Front Immunol. 2024. PMID: 38694505 Free PMC article.
-
Comparison of atezolizumab, durvalumab, pembrolizumab, and nivolumab as first-line treatment in patients with extensive-stage small cell lung cancer: A systematic review and network meta-analysis.Medicine (Baltimore). 2021 Apr 16;100(15):e25180. doi: 10.1097/MD.0000000000025180. Medicine (Baltimore). 2021. PMID: 33847617 Free PMC article.
-
Comparison of efficacy and safety of PD-1/PD-L1 combination therapy in first-line treatment of advanced NSCLC: an updated systematic review and network meta-analysis.Clin Transl Oncol. 2024 Oct;26(10):2488-2502. doi: 10.1007/s12094-024-03442-3. Epub 2024 Apr 16. Clin Transl Oncol. 2024. PMID: 38625495
-
Efficacy and safety of novel immune checkpoint inhibitor-based combinations versus chemotherapy as first-line treatment for patients with extensive-stage small cell lung cancer: A network meta-analysis.Thorac Cancer. 2024 May;15(15):1246-1262. doi: 10.1111/1759-7714.15310. Epub 2024 Apr 16. Thorac Cancer. 2024. PMID: 38623838 Free PMC article.
-
Efficacy and safety of first-line PD-1/PD-L1 inhibitors combined with or without anti-angiogenesis therapy for extensive-stage small-cell lung cancer: a network meta-analysis.Ther Adv Med Oncol. 2025 Jun 25;17:17588359251348310. doi: 10.1177/17588359251348310. eCollection 2025. Ther Adv Med Oncol. 2025. PMID: 40574965 Free PMC article.
Cited by
-
The efficacy and safety of immunotherapy as first-line treatment for extensive-stage small cell lung cancer: evaluating based on reconstructed individual patient data.Front Oncol. 2024 Jul 4;14:1371313. doi: 10.3389/fonc.2024.1371313. eCollection 2024. Front Oncol. 2024. PMID: 39026980 Free PMC article.
-
Navigating first-line therapies for extensive-stage small-cell lung cancer: a frequentist network meta-analysis and systematic review.Future Oncol. 2024;20(28):2109-2122. doi: 10.1080/14796694.2024.2376514. Epub 2024 Jul 29. Future Oncol. 2024. PMID: 39072397 Free PMC article.
-
Treatment patterns and clinical outcomes in 157 patients with extensive-stage small cell lung cancer: real-world evidence from a single-center retrospective study.Front Oncol. 2023 Dec 4;13:1287628. doi: 10.3389/fonc.2023.1287628. eCollection 2023. Front Oncol. 2023. PMID: 38111524 Free PMC article.
-
Liquid biopsy in diagnosis and monitoring of treatment efficacy in patients with small cell lung cancer.Mol Biol Rep. 2025 May 13;52(1):455. doi: 10.1007/s11033-025-10569-1. Mol Biol Rep. 2025. PMID: 40358752 Free PMC article. Review.
-
Efficacy and safety evaluation of first-line systemic treatments for unresectable esophageal squamous cell carcinoma: a network meta-analysis.Front Oncol. 2024 Sep 9;14:1397960. doi: 10.3389/fonc.2024.1397960. eCollection 2024. Front Oncol. 2024. PMID: 39314629 Free PMC article. Review.
References
-
- Micke P, Faldum A, Metz T, Beeh KM, Bittinger F, Hengstler JG, et al. . Staging small cell lung cancer: veterans administration lung study group versus international association for the study of lung cancer–what limits limited disease? Lung Cancer (2002) 37(3):271–6. doi: 10.1016/s0169-5002(02)00072-7 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

